4
Introducing Mini-implants to Your Clinical Practice
Patient consent
It is important that you feel comfortable with both your proposed treatment plan and the favourable risk–benefit ratio for mini-implants during the consent process. It is also very helpful to use patient-friendly terms, and to help educate patients by using a fixed appliance typodont model with one or several mini-implants in situ, and possibly clinical photographs of the proposed treatment scenario. When you are starting to use mini-implants in your clinical practice, it may be worth pre-preparing an explanatory sentence or two which can ‘roll off your tongue’, such as: ‘we wish to move your teeth in this direction and need an anchor point to help achieve this. This little anchor, known as a mini-implant, may sound a bit daunting, but it is easily fitted by numbing just a small patch of your gum. It is even easier to remove later in your treatment, but this means that about one in ten anchors doesn’t achieve full stability and comes loose itself. This isn’t painful or harmful, but it is a nuisance since the mini-implant then needs to be replaced after a few months.’
It is important to document that the treatment details, mini-implant usage and potential problems have been fully discussed, including whether a mini-implant information sheet has been given to the patient (which is very useful as a routine step). This does not necessarily require a separate mini-implant consent process and form, in the same way that several types of orthodontic appliances may be included within a single treatment plan description. Instead, this topic is included as an integral part of the overall treatment options, plan and consent process. In the same vein, where relevant it may be appropriate to charge private patients in terms of their whole treatment plan, rather than having a separate add-on fee for mini-implant anchorage. The exception to this may be if you are planning for a colleague to insert the mini-implant(s) on your behalf, in which case fees and liabilities will need to be pre-agreed by the involved clinicians.
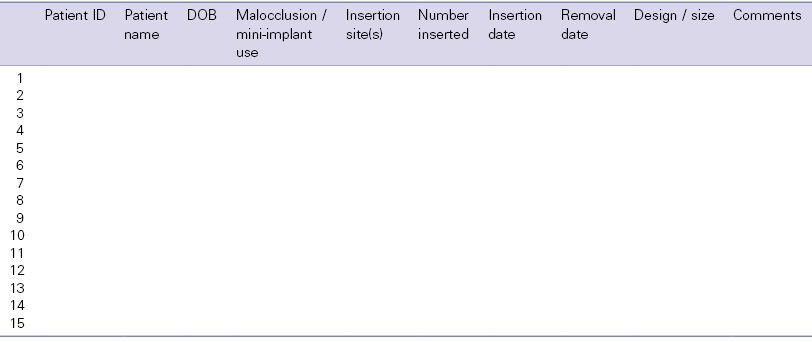
The main points to cover in written and verbal formats are listed below. It may not be appropriate to highlight all of these details to every patient, especially once you have experience and are able to discern those risks which are realistic or recurrent possibilities in your own clinical practice. A clinical audit of your caseload will provide valuable information and indicators of possible areas to focus on in this respect.1,2 This will also assist with clinical governance assurance. Such an audit is easy to conduct if you keep an up-to-date database of your mini-implant cases. A sample database spreadsheet, used for case compilation and audit, is shown in Table 4.1.
Table 4.1 A clinical database for mini-implant audit purposes.
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


