31 LUTING AGENTS AND CEMENTATION PROCEDURES
INTERIM CEMENTATION
On many occasions, cementing a restoration on an interim basis is advised so that the patient and dentist can assess its appearance and function over a time longer than a single visit. However, these trial cementations should be managed cautiously. On one hand, removing the restoration for definitive cementation may be difficult, even when temporary zinc oxide–eugenol (ZOE) cement is used. To avoid this problem, the interim cement can be mixed with a little petrolatum or silicone grease. The modified luting agent is applied only to the margins of the restoration to seal them and yet allow subsequent removal without difficulty. On the other hand, an interim cemented restoration may come loose during function. If a single unit is displaced, it can be embarrassing or uncomfortable for the patient. If one abutment of a partial fixed dental prosthesis (FDP) becomes loose, the consequences can be more severe. If the patient does not return promptly for recementation, caries may develop very rapidly. Interim cementation should not be undertaken unless the patient is given clear instructions about the objectives of the procedure, the intended duration of the trial cementation, and the importance of returning if an abutment loosens. If removing an interim cemented fixed prosthesis is difficult, the use of a crown-removal device such as the CORONAflex* (see Chapter 32) is recommended.
DEFINITIVE CEMENTATION
Conventional Cast Restorations
Definitive cementation often does not receive the same attention to detail as do other aspects of restorative dentistry. Careless luting agent selection can result in margin discrepancies and improper occlusion and may even necessitate cutting the restoration from the patient’s mouth and making a new one. The choice of cement depends first on whether a conventional casting or an adhesively bonded restoration, such as a ceramic inlay or resin-bonded partial FDP, is to be cemented. Traditional dental cements can be used for cast crowns and FDPs, but not where adhesion is needed. Adhesive resins are necessary for some restorations, but they can be difficult to use; in addition, there are no long-term data to justify more general use with conventional castings.
Dental Cements
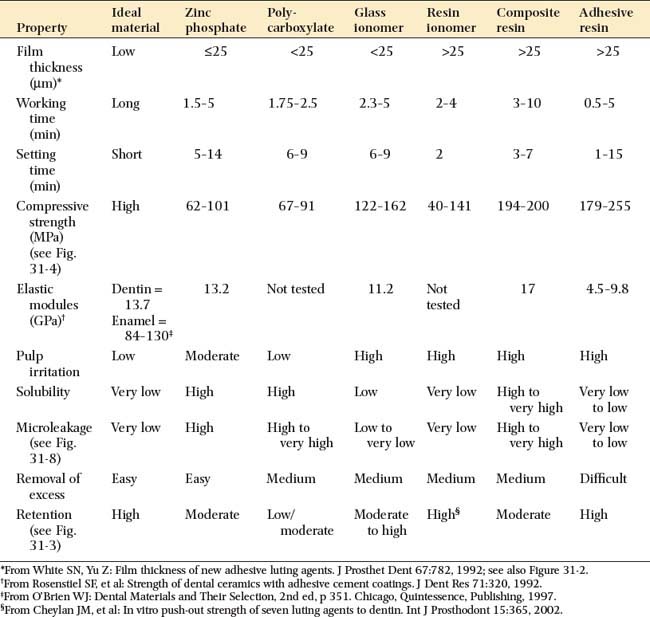
Most luting agents traditionally used for cast restorations are dental cements (Fig. 31-1). These consist of an acid combined with a metal oxide base to form a salt and water. The setting mechanism results from the binding of unreacted powder particles by a matrix of salt to harden the mass. However, because they are ionic, these agents are susceptible to acid attack and are therefore somewhat soluble in oral fluids.1–4 Traditionally, the success of restorations cemented with these luting agents has been attributed to excellent adaptation between the casting and the prepared tooth. In vitro, however, cement dissolution is independent of the marginal width up to a certain critical value. After that, it increases only slightly, which is explained by Fick’s first law of diffusion.*5
This study and others identify dissolution (rather than physical disintegration) as the mechanism for cement erosion.6 They explain the success of cast restorations, despite the prevalence of relatively large subgingival marginal discrepancies, which are difficult to detect even at 0.1 mm.7
Zinc phosphate cement
This traditional luting agent continues to be popular for cast restorations. It has adequate strength, a film thickness (thickness of the layer) of about 25 μm (Fig. 31-2) (which is within the tolerance limits required for making cast restorations),8 and a reasonable working time. Excess material can be easily removed.
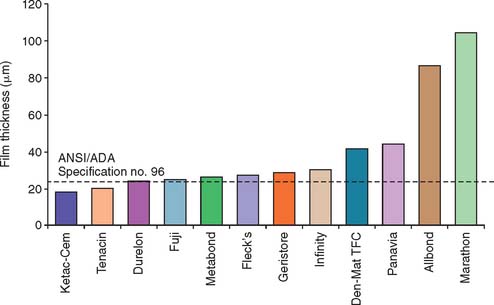
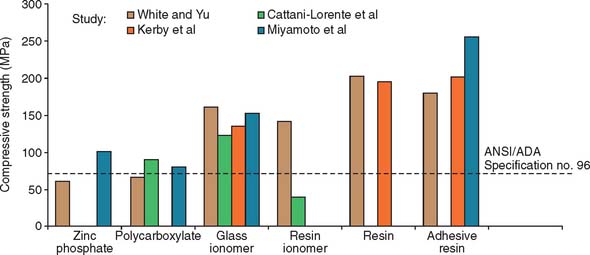
Fig. 31-2 The film thickness of a range of luting agents was tested according to American Dental Association (ADA) specification No. 8 for zinc phosphate cement (now American National Standards Institute [ANSI]/ADA specification No. 96) by White and Yu.64 Some of the adhesive materials possessed unacceptably high film thicknesses, which may translate into clinical problems for complete restoration seating.
(From Rosenstiel SF, et al: Dental luting agents: a review of the current literature. J Prosthet Dent 80:280, 1998.)
The toxic effects of zinc phosphate, or, more specifically, phosphoric acid, are well documented.9 However, the success of the use of this material over many years suggests that its effect on the dental pulp is clinically acceptable as long as normal precautions are taken and the preparation is not too close to the pulp.
Zinc polycarboxylate cement
One advantage of this luting agent is its relative biocompatibility,10 which may stem from the fact that the polyacrylic acid molecule is large and therefore does not penetrate into the dentinal tubule.
Zinc polycarboxylate cement also exhibits specific adhesion to tooth structure because it chelates the calcium (although it has no adhesion to gold castings). Because of its high viscosity, zinc polycarboxylate cement can be difficult to mix, but this problem can be overcome by using encapsulated products.*
In clinical trials, polycarboxylate performs as well as or slightly better than zinc phosphate.11,12 However, dentists have reported varying success rates, and claims of inferior long-term retention have been made. These problems may be related to the powder/liquid ratio. At manufacturers’ recommended powder/liquid ratios, mixed polycarboxylate cement is very viscous. Some dentists may prefer a more fluid working consistency for reliable seating during cementation. However, polycarboxylate cements have different rheologic or flow properties than zinc phosphate, exhibiting thinning with increased shear rate.13 This means that they are capable of forming low film thicknesses despite their viscous appearance. When the dentist unnecessarily reduces the powder/liquid ratio, the solubility (how susceptible something is to being dissolved) of the cement increases dramatically (as much as threefold).14 This may be the cause of increased clinical failures. By fabricating luting agents, including polycarboxylate in encapsulated form, manufacturers have reduced problems arising from manipulative variables.
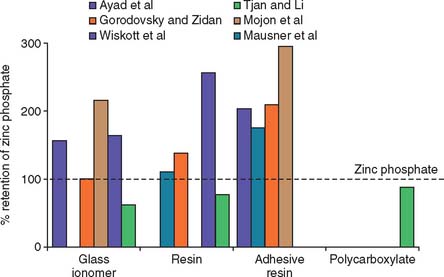
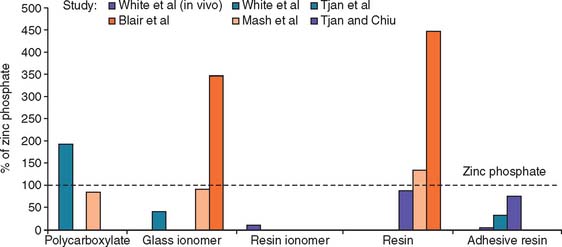
The working time of polycarboxylate is much shorter than that of zinc phosphate (about 2.5 minutes, in comparison with 5 minutes). This may be a problem when multiple units are being cemented. Residual zinc polycarboxylate is more difficult to remove than zinc phosphate, and there is some evidence15,16 that it provides less crown retention than zinc phosphate (Fig. 31-3). Its selection therefore should probably be limited to restorations with good retention and resistance form where minimum pulp irritation is wanted. Its use as a base material and to block out minor undercuts in preparations on vital teeth may also be worth considering.
Glass ionomer cement
This cement adheres to enamel and dentin and exhibits good biocompatibility. In addition, because it releases fluoride,17,18 it may have an anticariogenic effect, although this has not been documented clinically.19 The set cement is somewhat translucent, which is an advantage when it is used with the porcelain labial margin technique (see Chapter 24).
The mechanical properties of glass ionomer cement are generally superior compared with zinc phosphate or polycarboxylate cements (Fig. 31-4). A disadvantage is that during setting, glass ionomer appears particularly susceptible to moisture contamination20 and should be protected with a foil or resin coat or by leaving a band of cement undisturbed for 10 minutes.21 The water changes the setting reaction of the glass ionomer as cement-forming cations are flushed away and water is absorbed, which leads to erosion.22 Nevertheless, zinc phosphate has also demonstrated significant early erosion when exposed to moisture.18 Glass ionomers should not be allowed to desiccate during this critical initial setting period. The newer resin-modified glass ionomers are less susceptible to early moisture.23
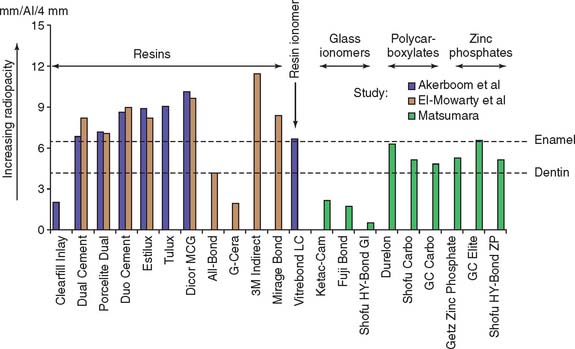
Although glass ionomers have been reported to cause sensitivity,24 there appears to be little pulpal response at the histologic level,25 particularly if the remaining dentin thickness exceeds 1 mm.26 Side effects such as posttreatment sensitivity thought to result from a lack of biocompatibility may actually be a result of desiccation or bacterial contamination27 of the dentin rather than irritation by the cement. The anecdotal reports that glass ionomer causes more post-treatment sensitivity have not been replicated in clinical trials. Authors have reported little association between the choice of zinc phosphate or glass ionomer cement and increased pulpal sensitivity, provided that manufacturers’ recommendations were followed28–30 (Fig. 31-5). If postcementation sensitivity becomes a problem, dentists should carefully evaluate their technique, particularly avoiding desiccation of the prepared dentin surface.31 Resin-modified glass ionomer materials have been reported to provoke less posttreatment sensitivity. Again, this information is anecdotal and has not been confirmed by clinical research.32 A desensitizing agent may prevent sensitivity, although it may also reduce retention, at least with some luting cements.16,33 Some formulations of glass ionomer and resin cements are radiolucent (Fig. 31-6), which may prevent the practitioner from distinguishing between a cement line and recurrent caries, as well as detecting cement overhangs.34 The use of a glass ionomer luting agent in general practice has been favorable35; however, any reduction in caries activity that might be anticipated by the fluoride content has not been demonstrated by clinical research.36
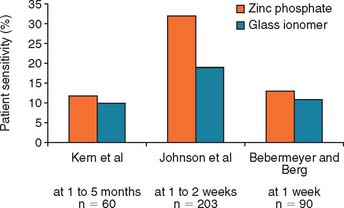
Fig. 31-5 Clinical trials28–30 that evaluated postcementation sensitivity of patients with crowns cemented with zinc phosphate or glass ionomer cement. Contrary to anecdotal evidence, those with glass ionomer cemented crowns did not exhibit increased postcementation sensitivity.
(From Rosenstiel SF, et al: Dental luting agents: a review of the current literature. J Prosthet Dent 80:280, 1998.)
Zinc oxide–eugenol with and without ethoxybenzoic acid
Reinforced ZOE cement is extremely biocompatible and provides an excellent seal. However, its physical properties are generally inferior to those of other cements, which limits its use.37 In terms of compressive strength, solubility, and film thickness, another luting agent (e.g., zinc phosphate) should be used. The ethoxybenzoic acid (EBA) modifier replaces a portion of the eugenol in conventional ZOE cement, although the change improves compressive strength without affecting its resistance to deformation; the cement should be used only in restorations with good inherent retention form in which emphasis is on biocompatibility and pulpal protection. The EBA cement has a relatively short working time, and excess material is difficult to remove.
Resin-modified glass ionomer luting agents
Resin-modified glass ionomers* were introduced in the 1990s in an attempt to combine some of the desirable properties of glass ionomer (i.e., fluoride release and adhesion) with the higher strength and low solubility of resins. These materials are less susceptible to early moisture exposure38 than is glass ionomer and are currently among the most popular materials in general practice. The materials exhibit higher strength than the conventional cements; values are similar to the resin luting agents.39 Resin-modified glass ionomers should be avoided with all-ceramic restorations because some brands have been associated with fracture,40,41 which is probably caused by their water absorption and expansion.42
Resin luting agents
Unfilled resins have been used for cementation since the 1950s. Because of their high polymerization shrinkage and poor biocompatibility, these early products were unsuccessful, although they had very low solubility. Composite resin cements with greatly improved properties were developed for resin-bonded prostheses (see Chapter 26) and are used extensively for the bonded ceramic technique (see Chapter 25). Resin cements are available with adhesive properties (i.e., they are capable of bonding chemically to dentin).43 Bonding is usually achieved with organophosphonates, hydroxyethyl methacrylate (HEMA), or 4-methacryloxyethyl-trimellitic anhydride (4-META).44 These developments, and their lack of solubility, have rekindled an interest in the use of resin cements for crowns and conventional fixed prostheses (Fig. 31-7). Resin luting agents are less biocompatible than cements such as glass ionomer, especially if they are not fully polymerized. They also tend to have greater film thickness.45
Choice of Luting Agent
An ideal luting agent has a long working time, adheres well to both tooth structure and cast alloys, provides a good seal, is nontoxic to the pulp, has adequate strength properties, is compressible into thin layers, has low viscosity and solubility, and exhibits good working and setting characteristics. In addition, any excess can be easily removed. Unfortunately, no such product exists (Tables 31-1 and 31-2).
Zinc phosphate cement
Despite its limited biocompatibility in terms of pulp irritation, zinc phosphate has a long history, and its limitations are well documented. This factor is important for cast restorations, which should be designed for long-term service. Zinc phosphate cement is probably still the luting agent of choice for otherwise normal, conservatively prepared teeth. Cavity varnish can be used to protect against pulp irritation from phosphoric acid and appears to have little effect on the amount of retention of the cemented restorations.46 In addition, crowns cemented with zinc phosphate displayed increased resistance to dislodgment on preparations lacking resistance form.31
Resin-modified glass ionomer luting agents
Currently among the most popular luting agents, resin-modified glass ionomer luting agents have low solubility, adhesion, and low microleakage* (Fig. 31-8). The popularity of these materials is derived mainly from the perceived benefit of reduced postcementation sensitivity, although this benefit has not been confirmed in clinical studies.47
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses