White Lesions
Hereditary Conditions
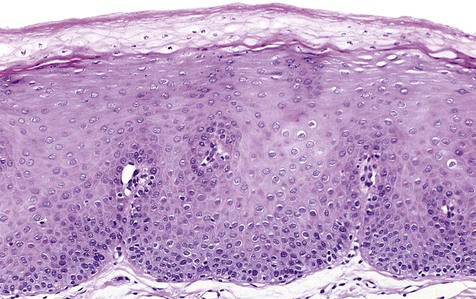
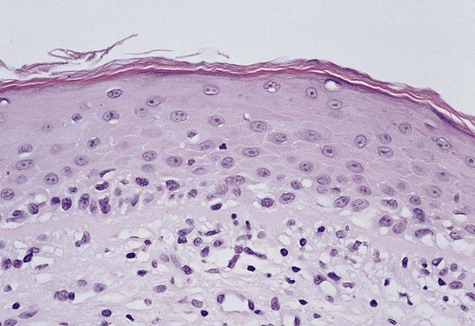
Leukoedema
Clinical Features
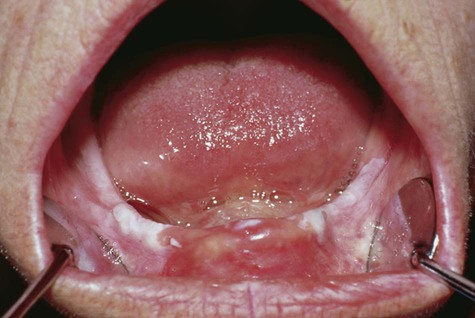
Leukoedema is usually discovered as an incidental finding. It is asymptomatic and symmetrically distributed in the buccal mucosa, and to a lesser extent over the labial mucosa. It appears as a gray-white, diffuse, filmy, or milky surface alteration (Fig. 3-1). In exaggerated cases, a whitish cast with surface textural changes, including wrinkling or corrugation, may be seen. With stretching of the buccal mucosa, the opaque changes dissipate. It is more apparent in nonwhites, especially African Americans.
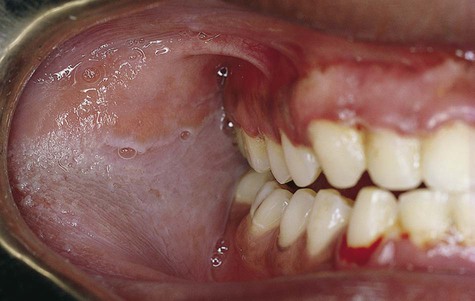
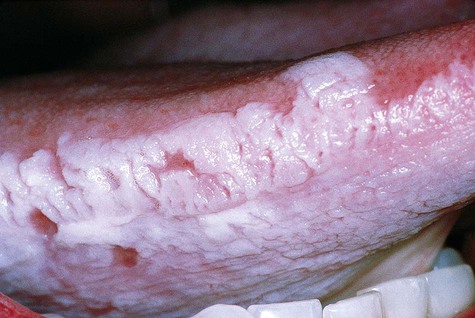
White Sponge Nevus
Clinical Features
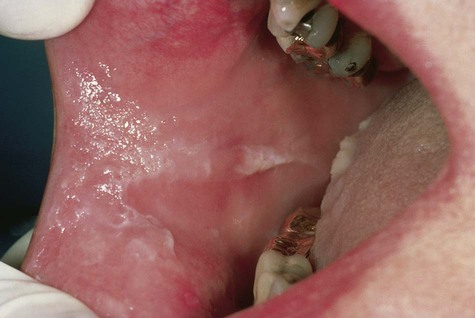
WSN presents as an asymptomatic, folded, white lesion that may affect several mucosal sites (Fig. 3-2; Box 3-1). Lesions tend to be thickened and have a spongy consistency. The presentation intraorally is almost always bilateral and symmetric and usually appears early in life, typically before puberty. The characteristic clinical manifestations of this particular form of keratosis are usually best observed on the buccal mucosa, although other areas such as the tongue and vestibular mucosa may also be involved. The conjunctival mucosa is usually spared, but mucosa of the esophagus, anus, vulva, and vagina may be affected.
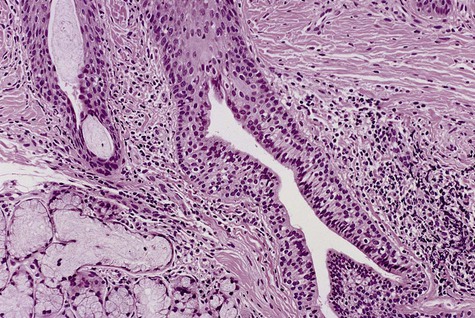
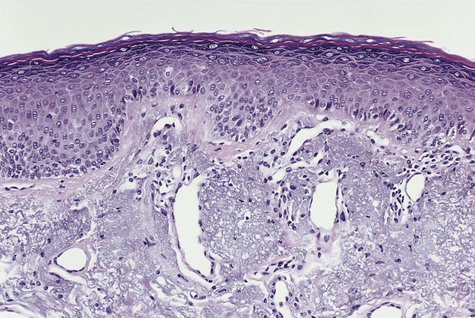
Histopathology
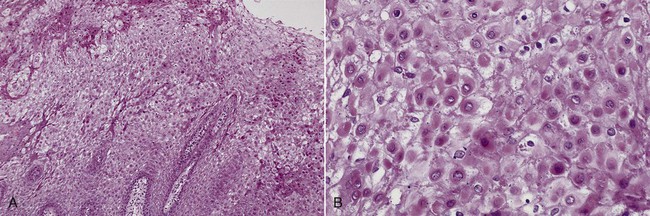
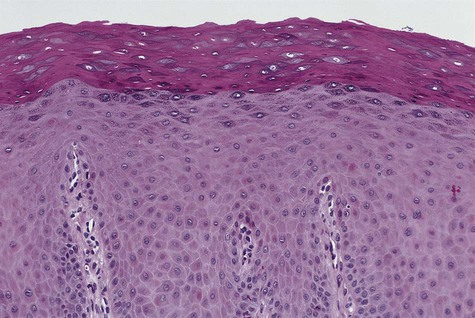
Microscopically, the epithelium is greatly thickened, with marked spongiosis, acanthosis, and parakeratosis (Fig. 3-3). Within the stratum spinosum, marked hydropic or clear cell change may be noted, often beginning in the parabasal region and extending very close to the surface. Perinuclear eosinophilic condensation of cytoplasm is characteristic of prickle cells in WSN. It is often possible to see columns of parakeratin extending from the spinous layer to the surface.
Differential Diagnosis
The differential diagnosis includes hereditary benign epithelial dyskeratosis, lichen planus, lichenoid drug reaction, lupus erythematosus (LE), cheek chewing, and possibly candidiasis (Table 3-1). Once tissue diagnosis is confirmed, no additional biopsies are necessary.
TABLE 3-1
BILATERAL BUCCAL MUCOSAL WHITE LESIONS: DIFFERENTIAL DIAGNOSIS
| Disease | Features/Action |
| White sponge nevus and HBID | Hereditary; does not disappear when stretched; biopsy to confirm; HBID may also involve conjunctiva |
| Lichen planus | Look for white reticulations (striae) and skin lesions; biopsy |
| Lichenoid drug reaction | Look for white lesions in context of new drug history |
| Cheek chewing | White shaggy lesions along occlusal plane or trauma sites |
| Lupus erythematosus | Delicate radiating striae; biopsy |
| Candidiasis | Look for predisposing factors; can rub off; responds to antifungal therapy |
Reactive Lesions
Focal (Frictional) Hyperkeratosis
Etiology
Clinical Features
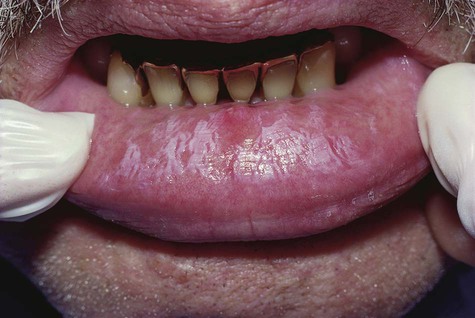
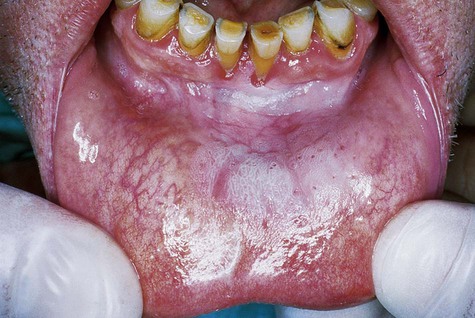
Friction-induced hyperkeratoses occur in areas that are commonly traumatized, such as the lips, lateral margins of the tongue, buccal mucosa along the occlusal line, and edentulous alveolar ridges (Figs. 3-4 to 3-7; Box 3-2). Chronic cheek or lip chewing may result in opacification (keratinization) of the affected area. Chewing on edentulous alveolar ridges produces the same effect.
Diagnosis
Careful history taking and examination should indicate the nature of this lesion. If the practitioner is clinically confident of a traumatic cause, no biopsy may be required. Patients should be advised to discontinue the causative habit, or the offending tooth or denture should be smoothed. The lesion should resolve or at least should be reduced in intensity over time, confirming the clinical diagnosis. Resolution of the lesion would allow unmasking of any underlying lesion that may not be related to trauma (Table 3-2). If the clinical diagnosis is in doubt, a biopsy should be taken.
TABLE 3-2
SOLITARY WHITE LESION: DIFFERENTIAL DIAGNOSIS
| Disease | Features/Action |
| Frictional keratosis | Look for cause (e.g., ill-fitting denture, trauma); biopsy |
| Dysplasia, in situ carcinoma, squamous cell carcinoma | Assess risk factors; biopsy |
| Burn | History of aspirin or other agent application at site of lesion—discontinue use |
| Lupus erythematosus | Delicate radiating striae; biopsy |
| Hairy leukoplakia | Lateral borders of tongue; look for irregular surface architecture; often bilateral; biopsy |
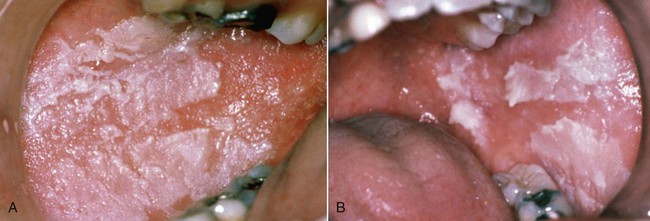
White Lesions Associated With Smokeless Tobacco
Clinical Features
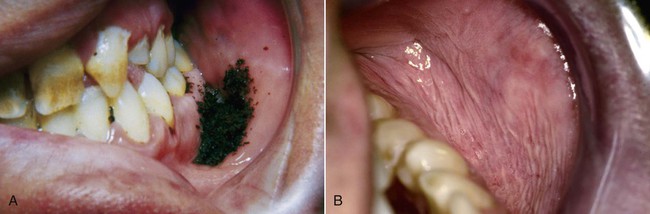
White lesions associated with smokeless tobacco develop in the immediate area where the tobacco is habitually placed (Figs. 3-9 and 3-10; Box 3-3). The most common area of involvement is the mucobuccal fold of the mandible in the incisor or the molar region. The mucosa develops a granular to wrinkled appearance. In advanced cases, a heavy, folded character may be seen. Less often, an erythroplakic or red component may be admixed with the white keratotic component. The lesions are generally painless and asymptomatic, and their discovery is often incidental to routine oral examination.
Histopathology
Slight to moderate parakeratosis, often in the form of spires or chevrons, is noted over the surface of the affected mucosa (Fig. 3-11). Superficial epithelium may demonstrate vacuolization or edema. A slight to moderate chronic inflammatory cell infiltrate is typically present. Epithelial dysplasia may occasionally develop in these lesions, especially among long-time users of smokeless tobacco. On occasion, a diffuse zone of basophilic stromal alteration may be seen, usually adjacent to inflamed minor salivary glands.
Nicotine Stomatitis
Etiology
Clinical Features
The palatal mucosa initially responds with an erythematous change followed by keratinization (Box 3-4). Subsequent to opacification or keratinization of the palate, red dots surrounded by white keratotic rings appear (Figs. 3-12 and 3-13). The dots represent inflammation surrounding the minor salivary gland excretory ducts.
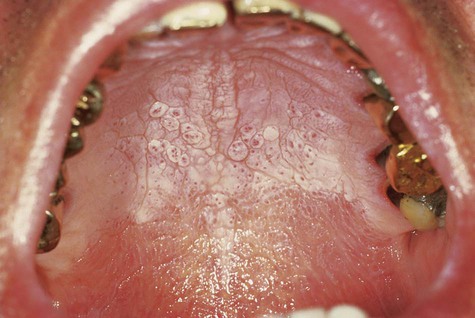
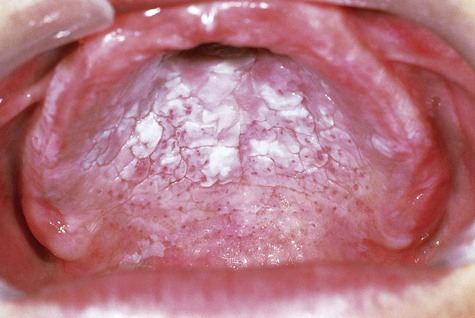
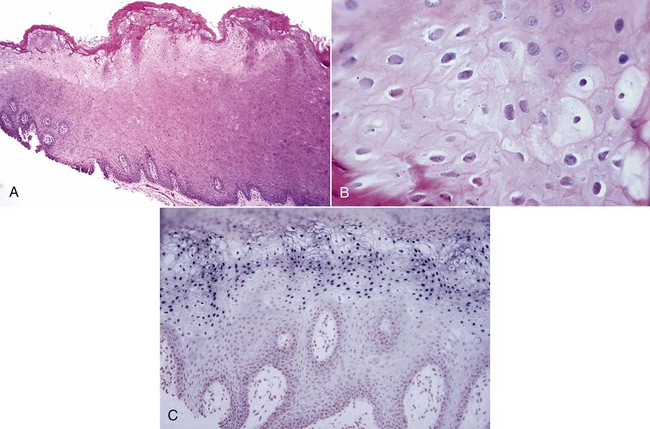
Hairy Leukoplakia
Etiology and Pathogenesis
The prevalence of hairy leukoplakia in HIV-infected patients has been declining as a result of new chemotherapeutic regimens for HIV. Of importance is that this lesion has been associated with subsequent or concomitant development of clinical and laboratory features of acquired immunodeficiency syndrome (AIDS) in as many as 80% of cases. A positive correlation with depletion of peripheral CD4 cells and with the presence of hairy leukoplakia has been noted. Several other oral conditions have been described as having greater than expected frequency in patients with AIDS (Box 3-5).
Clinical Features
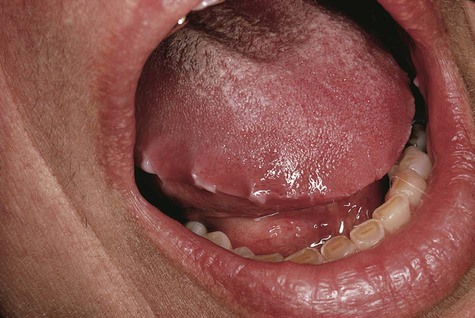
Hairy leukoplakia presents as a well-demarcated white lesion that varies in architecture from a flat and plaquelike to a papillary/filiform or corrugated lesion (Figs. 3-15 and 3-16; Box 3-6). It may be unilateral or bilateral. A vast majority of cases have been located along the lateral margins of the tongue, with occasional extension onto the dorsal surface. Rarely, hairy leukoplakia may be seen on the buccal mucosa, the floor of the mouth, or the palate. Lesions have not been seen in the vaginal or anal mucosa.
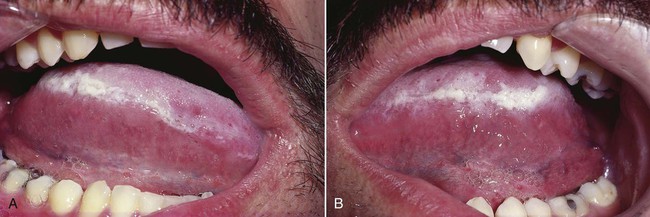
Histopathology
The characteristic microscopic feature of hairy leukoplakia is found in the nuclei of upper level keratinocytes (Fig. 3-17). Viral inclusions or peripheral displacement of chromatin with a resultant smudgy nucleus is evident. This is seen in the context of a markedly hyperparakeratotic surface, often with the formation of keratotic surface irregularities and ridges. C. albicans hyphae are often seen extending into the superficial epithelial cell layers. Beneath the surface, within the spinous cell layer, cells show ballooning degeneration and perinuclear clearing. A general paucity of subepithelial inflammatory cells has been noted, and Langerhans cells are scant.
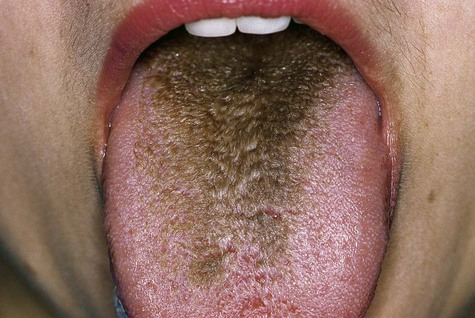
Hairy Tongue
Clinical Features
The clinical alteration translates to asymptomatic hyperplasia of the filiform papillae, with concomitant retardation of the normal rate of desquamation. The result is a thick, matted surface that serves to trap bacteria, fungi, cellular debris, and foreign material (Fig. 3-18; Box 3-7).
Dentifrice-Associated Slough
Dentifrice-associated slough is a relatively common phenomenon that has been associated with the use of several different brands of toothpaste. It is believed to be a superficial chemical burn or a reaction to a component in the dentifrice, possibly the detergent or flavoring compounds. This process may be related to the use of essential oil–containing mouth rinses. Clinically, it appears as a superficial whitish slough of the buccal mucosa, typically detected by the patient as oral peeling that easily swipes away (Fig. 3-19). The condition is painless and is not known to progress to anything significant. The problem resolves with a switch to another, blander toothpaste or mouth rinse.
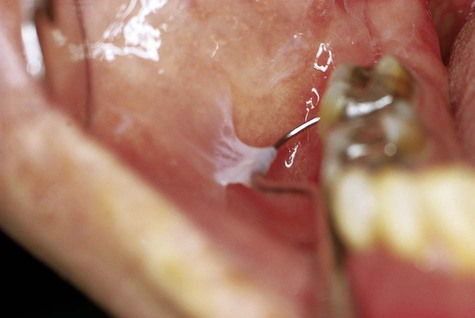
White mucosal changes have been described in association with the use of toothpaste and mouthwashes containing the substance sanguinaria (Fig. 3-20). The alteration is typically seen in the maxillary vestibule, although other sites may be affected.
Preneoplastic and Neoplastic Lesions
Actinic Cheilitis
Clinical Features
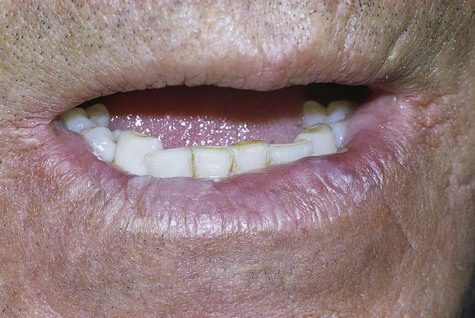
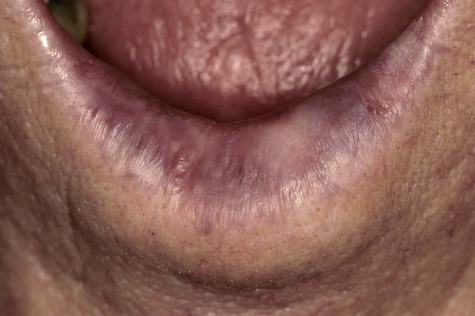
The affected vermilion of the lips takes on an atrophic, pale to silvery gray, glossy appearance, often with fissuring and wrinkling at right angles to the cutaneous-vermilion junction (Fig. 3-21; Box 3-8). Slightly firm, bilateral swelling of the lower lip is common. In advanced cases, the junction is irregular or totally effaced, with a degree of epidermization of the vermilion. Mottled areas of hyperpigmentation and keratosis are often noted, as well as superficial scaling, cracking, erosion, ulceration, and crusting (Fig. 3-22).
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses