Preventive Dental Materials
Objectives
After reading this chapter, the student should be able to:
Fluoride, Gels, Rinses, and Varnishes
1. Indicate the components in fluoride gels, rinses, and varnishes.
< ?mpslid E1?>< ?mpslid S2?>
2. Compare the characteristics of different types of fluoride treatments.
< ?mpslid E2?>< ?mpslid S3?>
3. Describe the clinical effectiveness of fluoride gels.
< ?mpslid E3?>< ?mpslid S4?>
4. Give the pH range of many commercial fluoride gels.
< ?mpslid E4?>< ?mpslid S5?>
5. List five steps involved in the application of a fluoride gel.
< ?mpslid E5?>
Pit and Fissure Sealants
1. Describe the uniqueness of pit and fissure caries compared with smooth-surface caries.
< ?mpslid E6?>< ?mpslid S7?>
< ?mpslid E7?>< ?mpslid S8?>
3. Describe factors that affect the penetration of a sealant into a fissure.
< ?mpslid E8?>< ?mpslid S9?>
4. Discuss the retention and efficacy of sealants.
< ?mpslid E9?>< ?mpslid S10?>
5. Describe the clinical success of sealants.
< ?mpslid E10?>< ?mpslid S11?>
6. List four situations in which sealant should not be used.
< ?mpslid E11?>< ?mpslid S12?>
7. List six steps involved in the application of sealants.
< ?mpslid E12?>
Mouth Protectors
1. Give the percentage of oral injuries sustained in unorganized sports.
< ?mpslid E13?>< ?mpslid S14?>
2. List common reactions of teeth to trauma.
< ?mpslid E14?>< ?mpslid S15?>
< ?mpslid E15?>< ?mpslid S16?>
4. Compare the characteristics of different types of mouth protectors.
< ?mpslid E16?>< ?mpslid S17?>
5. List eight physical and mechanical properties that characterize a mouth-protector material.
< ?mpslid E17?>< ?mpslid S18?>
6. List eight properties of a mouth protector that can be evaluated clinically.
< ?mpslid E18?>< ?mpslid S19?>
7. Discuss the clinical implications of the properties of hardness and tearing.
< ?mpslid E19?>< ?mpslid S20?>
8. Describe three causes of breakdown of a mouth protector.
< ?mpslid E20?>< ?mpslid S21?>
< ?mpslid E21?>< ?mpslid S22?>
< ?mpslid E22?>< ?mpslid S23?>
11. Indicate two goals in the forming of a mouth protector.
< ?mpslid E23?>< ?mpslid S24?>
12. Give two mistakes common in the fabrication of a mouth protector.
< ?mpslid E24?>< ?mpslid S25?>
13. List five instructions to give to a patient for the proper care of a mouth protector.
< ?mpslid E25?>
Key Terms
Abrasion
Alumina
Amine
Bisphenol A-glycidyl methacrylate (Bis-GMA)
Ceramics
Compomers
Composites
Cross-linked polymer
Dimethacrylate
Gel
Glass ionomers
Hybrid ionomers
Initiator
Monomer
Palatal area
Polymerization
Poly(vinyl acetate)-polyethylene
Polyurethane
Resin-modified glass ionomers
Thermoplastic
Thixotropic
Varnish
Preventive dental materials are designed to prevent disease or injury to the teeth and supporting tissues. Three preventive materials are fluoride gels or varnishes, pit and fissure sealants, and mouth protectors. Fluoride gels are applied in a tray to the teeth after a dental prophylaxis or at home to prevent smooth-surface caries. Fluoride rinses and varnishes are also available. Pit and fissure sealants are polymers applied to the occlusal surfaces of posterior teeth to prevent pit and fissure caries. Mouth protectors are made from polymers formed by heat to fit over the teeth of the maxillary arch to protect the mouth from sudden blows that could fracture or dislodge the teeth. Mouth protectors also may be used as trays, or carriers, to provide topical fluoride or bleaching applications, or as shields to prevent damage from bruxism.
Fluoride Gels, Foams, Rinses, and Varnishes
Numerous clinical studies have established the effectiveness of the fluoride ion in lowering the incidence of dental caries. Methods to accomplish topical application of fluoride are the use of gels in trays, rinses, and varnishes.
Composition
Typical commercial acidulated phosphate-fluoride (APF, 12,300 ppm fluoride) gels contain 2% sodium fluoride, 0.34% hydrogen fluoride, and 0.98% phosphoric acid with thickening, flavoring, and coloring agents in an aqueous gel. Some commercial gels, however, contain more sodium fluoride (2.6%) but less hydrogen fluoride (0.16%). The fluoride-ion concentration of most gels ranges from 1.22% to 1.32%. Examples of office and prescription fluoride treatments are listed in Table 3-1. APF products are contraindicated for patients with tooth hypersensitivity—they can cause erosion and worsen the hypersensitivity.
TABLE 3-1
Examples of Office and Prescription Fluoride Treatments
< ?comst?>
| FLUORIDE DELIVERY SYSTEM | TYPE | CONCENTRATION | PRODUCT | MANUFACTURER |
| Acidulated phosphate-fluoride | Office-use foam | 1.23% | ALLSolutions Fluoride Foam | DENTSPLY Professional (York, PA) |
| Sodium fluoride | Office-use foam | 2.0% | Oral-B Neutra-Foam | Oral-B (South Boston, MA) |
| Home-use rinse | 0.2% | PreviDent Rinse | Colgate Professional (Canton, MA) | |
| Stannous fluoride | Home-use gel | 0.4% | Perfect Choice | Challenge Products (Louisville, CO) |
< ?comen?>< ?comst1?>
< ?comst1?>
< ?comen1?>
Neutral sodium fluoride foams, gels, and rinses are available. One product is thixotropic, which contains sodium fluoride and thickening agents (polyacrylic acid and a gum). The pH is adjusted to between 6 and 8. Values of pH in this range should minimize acid etching of restorative materials, such as composites, compomers, resin-modified glass ionomers (hybrid ionomer), glass ionomers, and ceramics, caused by more acidic APF gels.
Varnishes containing 5% sodium fluoride (22,600 ppm fluoride) are available (Duraflor Halo 5% Sodium Fluoride White Varnish, Medicom, Tonawanda, NY; Kolorz ClearShield Fluoride Varnish, DMG America, Englewood, NJ). Some products (Enamel Pro Varnish, Premier Dental Products, Plymouth Meeting, PA) also contain amorphous calcium phosphate (ACP), which contributes to remineralization of enamel.
Stannous fluoride products are effective in providing fluoride but can cause staining of tooth surfaces and restorations.
Properties
Characteristics of different types of fluoride treatments are compared in Table 3-2. The clinical effectiveness of acidulated phosphate-fluoride gels varies, which depends in part on the method and frequency of application. Reductions in dental caries of 37% and 41% were observed in two studies of 2 years’ duration in which the gel was applied annually. A reduction of 26% was observed at the end of 3 years in another study. A reduction of 80% after 2 years was observed in a study in which a gel with lower fluoride content (0.5% versus 1.23%) and higher pH (pH 4.5 versus pH 3) than that used in the aforementioned studies was self-applied each school day. One clinical study showed no significant reduction in the incidence of dental caries after 2 years. The typical 4-minute application appears to be more effective than a 1-minute application.
TABLE 3-2
Characteristics of Different Types of Fluoride Treatments
< ?comst?>
| CHARACTERISTIC | ACIDULATED PHOSPHATE-FLUORIDE | SODIUM FLUORIDE | STANNOUS FLUORIDE |
| Form acidity (pH) | Gel, rinse, foam Acidic |
Gel, rinse, foam Neutral |
Gel, rinse Acidic |
| Can etch restorations | Yes | No | Yes |
| Can stain restorations | No | No | Yes |
< ?comen?>< ?comst1?>
< ?comst1?>
< ?comen1?>
Varnish containing 5% sodium fluoride has been found to be effective in reducing caries in primary and permanent dentition. Caries reduction (Eecayed, Missing, Filled Surfaces – DMFS) ranged from 19% to 48% in primary dentition and from 30% to 63% in permanent dentition. Five percent sodium fluoride varnish is also effective in reducing orthodontic decalcification.
Manipulation
Fluoride foams and gels can be applied in soft, spongy trays after a dental prophylaxis. The teeth are kept as free from saliva as possible before application of the tray. A ribbon of gel is placed in the troughs of the maxillary and mandibular trays. Then the trays are placed in position, and pressure is applied by squeezing the buccal and lingual surfaces to mold the tray tightly around the teeth so that the gel penetrates between the teeth. The patient is instructed to bite lightly for 4 minutes. After application of a gel, the patient is instructed not to eat for 30 minutes. Rinses are not recommended for children younger than 6 years.
Pit and Fissure Sealants
Smooth-surface caries has been reduced by the use of established preventive measures such as fluoridation of communal water supplies, topical application of fluoride during enamel development, and individual plaque-control programs. These measures, however, have not been completely effective in reducing the incidence of dental caries in pits and fissures, which are sites susceptible to dental caries because of their anatomic construction.
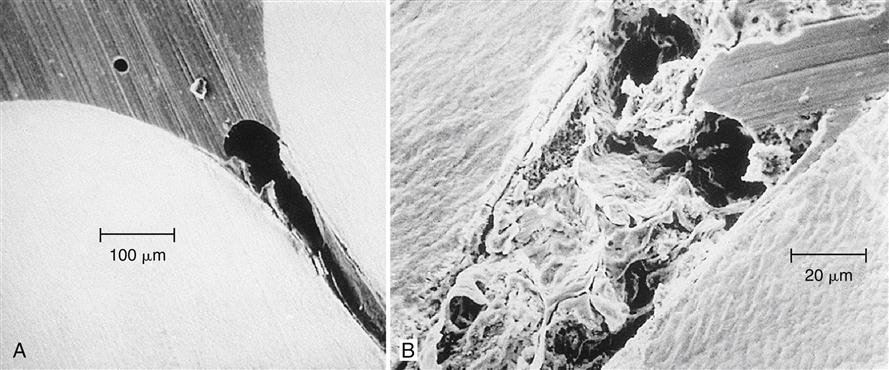
The uniqueness of pit and fissure caries is a result of the special anatomy of the occlusal surfaces of posterior teeth. A smooth-based depression on the occlusal surface of a tooth is termed a groove, an example of which is shown in a histologic section in Figure 3-1, A. The tip of an explorer in the upper left corner of this figure indicates the relative size of such a groove. A groove is cleansed by the excursion of food or of a toothbrush bristle. The pit and fissure, however, is an enamel fault that is the result of noncoalescence of enamel during tooth formation. This lack of enamel coalescence may extend to the dentoenamel junction, or it may be incomplete, with the fissure extending some lesser depth into the enamel. The debris and microbial masses that collect in a fissure are readily apparent in Figure 3-1, B. Under appropriate conditions, pit and fissure caries is initiated. The unusual anatomy of the pit and fissure causes such sites to exhibit a high incidence of dental caries. In fact, 84% of dental caries in children 5 to 17 years of age involve pits.
One approach to the prevention of pit and fissure caries has been a restorative procedure in which occlusal fissures are cut away and filled with dental amalgam. Another approach is the use of pit and fissure sealants. The purpose of a pit and fissure sealant is to penetrate all cracks, pits, and fissures on the occlusal surfaces of both deciduous and permanent teeth in an attempt to seal off these susceptible areas and to provide effective protection against caries. If incipient caries are suspected in pit and fissures of a tooth, the fissures are commonly prepared with small traditional carbide burs, specialized burs called fissurotomy burs, or by air abrasion (blasting with alumina [aluminum oxide] particles). The prepared fissures are then filled with a combination of flowable composite, traditional composites, or sealants, depending on the depth of the fissure preparation. This technique is called a “preventive resin restoration.”
Composition and Reaction
Most commercial pit and fissure sealants, examples of which are listed in Table 3-3, are resins in which polymerization is activated by light. The chemistry of sealants is similar to that of the composite restorative materials that are discussed in Chapter 4. The principal difference is that sealants are more fluid to penetrate the pits and fissures in addition to the etched areas produced on the enamel, which provide for retention of the sealant.
TABLE 3-3
Examples of Light-Cured Pit and Fissure Sealants
| PRODUCT | MANUFACTURER |
| Clinpro Sealant | 3M ESPE (St. Paul, MN) |
| Helioseal Clear Chroma | Ivoclar Vivadent (Amherst, NY) |
| Teethmate F-1 | Kuraray America (New York, NY) |
Sealants polymerized by visible light (490-nm wavelength) are one-component systems that require no mixing. The resin is a diluted dimethacrylate monomer (bisphenol A-glycidyl methacrylate [Bis-GMA] or urethane dimethacrylate [UDMA]), the polymerization of which is initiated by activation of a diketone in the presence of an organic amine with the visible light. Several sealants contain up to 50% by weight of inorganic filler to improve durability, and many contain a white pigment to improve the contrast between the sealants and enamel. The sealants polymerize in the mouth when exposed to a curing light to become a cross-linked polymer, as indicated in the following simplified reaction:
< ?xml:namespace prefix = "mml" ns = "http://www.w3.org/1998/Math/MathML" />
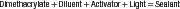
The sealants polymerized by an organic amine accelerator are supplied as two-component systems. One component contains a monomer and a benzoyl peroxide initiator, and the second component contains a diluted monomer with 5% organic amine accelerator. The two components are mixed thoroughly before being applied to the prepared teeth.
Properties
Physical and mechanical properties of commercial pit and fissure sealants are listed in Table 3-4. Additional properties of clinical importance include retention and efficacy.
TABLE 3-4
Properties of Resin Pit and Fissure Sealants
| PROPERTY | TYPICAL LIGHT-CURED SEALANT |
| Setting time (seconds) | Activated by light |
| Compressive strength (MPa) | 92–150 |
| Tensile strength (MPa) | 20–31 |
| Elastic modulus (GPa)∗ | 2.1–5.2 |
| Knoop hardness (kg/mm2) | 20–25 |
| Water sorption, 7 days (mg/cm2) | 1.3–2.0 |
| Water solubility, 7 days (mg/cm2) | 0.2 |
| Penetration coefficient, 22°C (cm/sec) | 4.5–8.8 |
| Wear (× 10-4 mm3/mm) | 22–23 |
Retention of a sealant in a fissure is the result of mechanical bonding caused by penetration of the sealant into the fissure and the etched areas of enamel to form tags. Filling the fissure completely is difficult because air frequently is trapped in the bottom of the fissure (Figure 3-2, A), or the accumulation of debris at the base of the fissure prevents it from being sealed completely (Figure 3-2, B). Acid etching of the enamel surface improves the retention of the sealant by cleaning the area to be sealed, improving the wettability of the enamel, increasing the surface area, and forming spaces into which the sealant can penetrate to form tags (Figure 3-3).
Penetration of a sealant into the fissure must occur before the sealant has polymerized. The rate of penetration is determined by the configuration (length and radius) of the pit or fissure and by the penetration coefficient (PC) of the sealant (see more details in Appendix 3-1). The penetration coefficient is related to the surface tension and viscosity of the sealant and the contact angle of the sealant on the enamel.
The containers in which the sealant components are supplied must be kept closed tightly during storage to minimize the evaporation of volatile monomers, which would cause the sealant to become more viscous and limit its penetration into the pit or fissure.
Many clinical studies have been reported. However, caution is warranted when comparing some of these studies because materials, techniques, teeth studied, and clinical criteria for judging success or failure vary from study to study. Three parameters important in the evaluation of a clinical study of a sealant are (1) a statistical test of the significance, (2) the net gain as a result of treatment, and (3) the percentage of effectiveness. When pairs of teeth are studied, the net gain is the number of pairs in which the treated tooth is sound and the untreated tooth is decayed minus the number of pairs in which the treated tooth is decayed and the untreated tooth is sound. The percentage of effectiveness is the net gain divided by the total number of carious controls expressed as a percentage. A summary of a 5-year clinical study on schoolchildren is listed in Table 3-5. The effectiveness of a single application of a sealant clearly decreases with time.
TABLE 3-5
Summary of a Clinical Study after Single Application of a Pit and Fissure Sealant
< ?comst?>
| TYPE OF TEETH AND PATIENTS | DURATION OF STUDY (YEARS) | TOTAL RETENTION (%) | NET GAIN (NUMBER OF TEETH) | EFFECTIVENESS (%) |
| First permanent molars in children 5 to 9 years of age | 1 | 79 | 77 | 83 |
| 2 | 71 | 96 | 74 | |
| 3 | 60 | 91 | 64 | |
| 4 | 52 | 78 | 54 | |
| 5 | 31 | 58 | 40 | |
| Second primary molars in children 5 to 9 years of age | 1 | 72 | — | — |
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses