3 Potential for Bleeding
3.0 INTRODUCTION
Dental patients may be at risk for abnormal peri- and post-operative bleeding due to a variety of inherited and acquired conditions that affect normal hemostasis. First and foremost in assessing this risk is obtaining a comprehensive medical and medication history, and patients should be specifically asked if they have bleeding or healing problems: Do you bleed or bruise easily? Do you have trouble with prolonged bleeding if you accidentally cut yourself? Do you have trouble with frequent nosebleeds? In patients who are considered to be at risk, appropriate ordering and interpretation of laboratory tests is warranted in most cases to evaluate the potential for bleeding complications. In some situations, abnormal or prolonged bleeding following a dental surgical procedure may be the initial presentation of an underlying bleeding disorder.
Hemostasis can be divided into four phases: (1) vascular, (2) platelet aggregation, (3) coagulation cascade, and (4) fibrinolysis. Following tissue injury, localized vasoconstriction minimizes blood loss. Exposure of the damaged vasculature activates platelets, which aggregate and rapidly form a temporary platelet plug. These early steps initiate the coagulation cascade, leading to fibrin formation that strengthens the initial clot. This is followed by fibrinolysis, in which the clot is degraded proteolytically, a necessary step for normal healing. Primary hemostasis is mediated primarily by adequate numbers of functioning platelets and occurs within minutes. Secondary hemostasis depends on normal levels of coagulation factors and takes place over hours to days. Rarely, underlying disorders in collagen and vessel wall integrity can result in clinically significant bleeding. These include metabolic and vitamin deficiencies (e.g., scurvy), collagen vascular disorders (e.g., Ehlers-Danlos syndrome, hereditary hemorrhagic telangiectasia), and endocrine disorders (e.g., Cushing syndrome). The majority of bleeding disorders are due to quantitative and qualitative abnormalities with platelets or coagulation proteins. Depending on the specific diagnosis and severity of abnormalities, bleeding can generally be anticipated prior to performing dental procedures, and measures must be taken to minimize risk of excess blood loss and the need for post-operative emergency management.
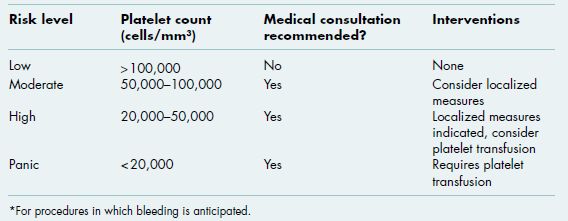
Figure 3.1 Multiple palatal petechiae and ecchymoses in a patient with severe thrombocytopenia secondary to aplastic anemia.
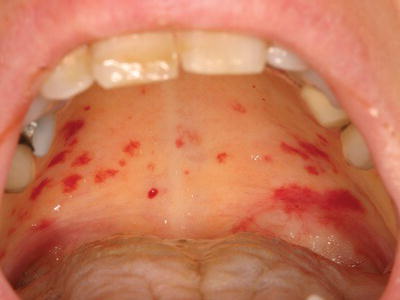
Figure 3.2 Petechiae of the marginal gingiva in a patient with severe thrombocytopenia secondary to acute leukemia.
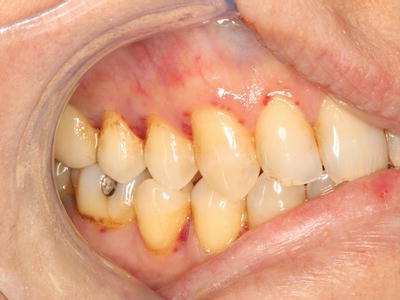
Oral soft tissue lesions, including petechiae (small red extravasation lesions), ecchymoses (larger extravasation lesions), and hematomas (exophytic rubbery lesions composed of clotted blood), are frequently encountered in patients with clinically significant bleeding disorders (Figures 3.1–3.4). The tongue, buccal mucosa, and soft palate are commonly affected due to normal functional tissue contact. These lesions are usually painless and asymptomatic, and all lesions, even large hematomas, will resolve spontaneously with correction of the underlying abnormality. Of note, all of the above lesions can develop secondary to trauma even in the absence of an underlying bleeding disorder, although they are not observed at nearly the same frequency.
Figure 3.3 Diffuse ecchymosis of the right buccal mucosa in an anticoagulated patient following an accidental fall.
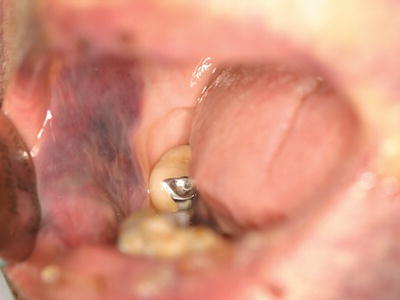
Figure 3.4 Exophytic hematoma of the right buccal mucosa in a patient with severe thrombocytopenia associated with advanced multiple myeloma.
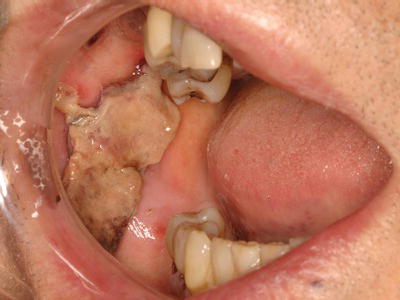
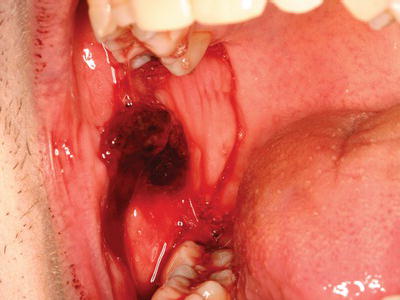
Dental procedures that involve any type of soft or hard tissue damage can potentially cause bleeding. The highest-risk procedures include dentoalveolar surgery, in particular multiple extractions, periodontal surgical procedures, including deep scaling and root planing, and soft tissue biopsies (Figure 3.5). Lower risk procedures in which excessive bleeding is rarely encountered include rubber dam clamp placement, use of retraction cord and local anesthesia injections. However, in patients with severe coagulopathies, even a simple inferior alveolar nerve block can induce life-threatening hematoma formation. Specific considerations are highlighted in this chapter.
Figure 3.5 Persistent bleeding following a punch biopsy in a patient with previously undiagnosed Factor XI deficiency.
3.1 Platelets
Platelets are generated from megakaryocytes in the bone marrow, circulate in the blood, and become activated when they come into contact with an injured blood vessel. An adequate number of circulating functional platelets are critical for primary hemostasis. In addition to exposure to the damaged vessel matrix, platelets also require the presence of von Willebrand factor (vWF), a plasma protein that facilitates initial platelet adhesion and stabilizes Factor VIII (FVIII; section 3.2.2.2.2). Following adhesion, platelets degranulate and release a number of intracellular components, including the potent nucleotide adenosine diphosphate (ADP), that recruit additional platelets, mediate aggregation, and initiate coagulation. Patients who are profoundly thrombocytopenic (less than 20,000 cells/mm3; Alert Box 3.1) are at significant risk for spontaneous bleeding and are often supported with platelet transfusions to maintain a minimal level of 20,000 cells/mm3.
3.1.1 Platelet tests
The most important test of primary hemostasis is the platelet count. Any patient suspected of having a decreased number of circulating platelets should have a platelet count obtained prior to any invasive surgical procedures. Several platelet function tests are available, but these are not widely used and should generally be ordered by a consulting hematologist when indicated.
3.1.1.1 Platelet count
Normal platelet counts range from 150,000 to 450,000 cells/mm3 (Alert Box 3.1). While there is large variation among the population, an individual’s count tends to remain within a certain range. The platelet count is purely quantitative and does not measure platelet functionality.
3.1.1.2 Bleeding time
The bleeding time is a fairly crude test of platelet function in which the skin is incised and observed for primary hemostasis in a standardized manner. This test is a poor indicator of mucosal and oral surgery induced bleeding and is therefore of limited clinical utility in dentistry.
3.1.1.3 Liver function tests
Since patients with advanced liver disease may present with both quantitative and qualitative platelet disorders in addition to clotting defects, liver function tests may provide additional information regarding an individual patient’s risk of bleeding (see chapter 2). Ordering of liver function tests for evaluation of primary hemostasis should be driven by patient history. The utilization of these tests for evaluation of secondary hemostasis is discussed below (section 3.2.2.2.3).
3.1.2 Platelet abnormalities
Conditions that lead to a decreased number of platelets, called thrombocytopenia, or dysfunctional platelet activity can cause abnormal bleeding. Thrombocytopenia may be due to decreased production, increased splenic sequestration, or accelerated destruction of platelets, while etiologies of dysfunctional platelet activity include medications, von Willebrand disease (vWD), and hematologic malignancies. These patients may present with gingival bleeding, excessive perioperative bleeding, or persistent post-operative bleeding due to insufficient primary hemostasis. Management is highly dependent on the nature and severity of the underlying condition as well as the planned procedure for which bleeding is anticipated.
3.1.2.1 Medication-induced
Certain medications have antiplatelet activity and are used therapeutically for management and prevention of thromboembolic disease (Table 3.1). However, many patients also take these medications for other reasons and may be unaware of their increased risk for bleeding. Risk assessment requires a careful medication history including reason for taking the medication, an understanding of the different mechanisms of action of the various antiplatelet medications, and use and interpretation of appropriate laboratory tests when indicated.
Table 3.1 Medications administered in the outpatient setting that may increase the risk of oral bleeding.
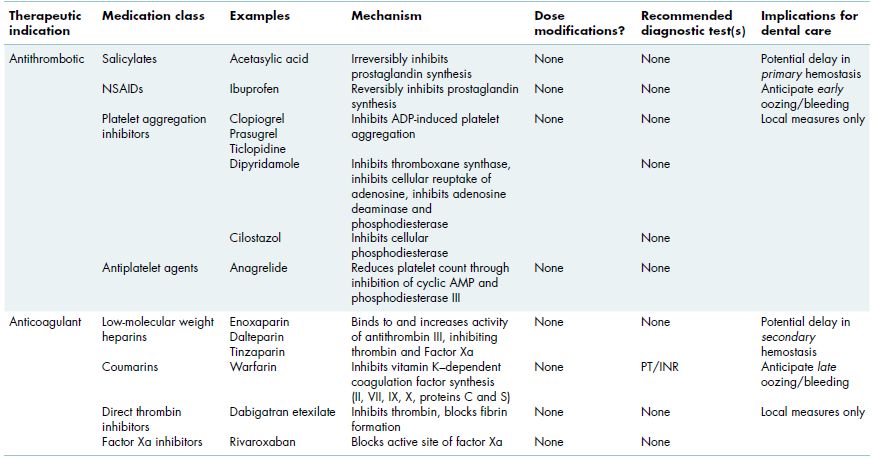
3.1.2.1.1 Acetasylic acid and non-steroidal anti-inflammatory agents
Acetasylic acid (ASA) is one of the most commonly prescribed medications used for thromboembolic prophylaxis and is also taken routinely for its analgesic properties. ASA inhibits prostaglandin synthetase, which blocks cyclooxygenase activity, resulting in decreased production of prostaglandins and thromboxanes, both of which are required for platelet function. This effect is irreversible and lasts 7–10 days, the lifetime of a platelet. Most of the non-steroidal anti-inflammatory agents (NSAIDs) have a similar but reversible effect, with complete restoration of platelet function within 3 days of medication discontinuation. NSAIDs that specifically inhibit cyclooxygenase 2 (COX2 inhibitors, e.g., celecoxib) rather than cyclooxygenase 1 (COX1, e.g., ibuprofen, naproxen) have minimal impact on platelet aggregation and bleeding risk.
3.1.2.1.2 ADP inhibitors
Clopidogrel bisulfate (Plavix) inhibits platelet aggregation by blocking ADP and is increasingly being used, often long term, for prevention of thromboembolic disease or prophylaxis following coronary artery stent placement. Prasurgel (Effient) is another ADP inhibitor that is Food and Drug Administration (FDA) approved for use in patients with acute coronary syndrome. Since the number of circulating platelets is not affected, the platelet count is not a useful test in patients taking these medications. Numerous studies have demonstrated that in almost all cases, dental surgical procedures, including multiple extractions, can be safely performed using basic localized hemostatic measures (section 3.2.3).
3.1.2.1.3 Chemotherapy agents
Cytoreductive chemotherapy agents often cause bone marrow suppression and a subsequent decrease in white blood cells, as well as platelets, due to destruction of rapidly dividing hematopoietic stem cells. The platelet count typically drops 6–10 days after initiation of therapy and restores gradually over a 1- to 2-week period after completion of therapy. Some agents and specific regimens are more likely to cause significant thrombocytopenia than others. Patients on multiple cycles of chemotherapy may be thrombocytopenic/>
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses