Materials and drugs
Few of the dental materials or drugs commonly used cause significant interactions or adverse reactions (see Appendices). The main concern is allergies (e.g. especially to latex [Ch. 17] and some drugs). Other issues include adverse reactions to dental materials (especially amalgam/mercury, chlorhexidine, nickel, iodides and radiocontrast media, resins, titanium), fluorides and anaesthetics – and other drug adverse effects, oral adverse effects, drug interactions and the effects of foods on drugs.
Dental Materials
Allergies are discussed in Chapter 17 and also below, alphabetically. Possible toxic effects of mercury on the nervous system are discussed here and in Chapter 17; fluoride is also discussed in this chapter.
Allergies
Latex allergy
Latex allergy (see also Ch. 17) is one of the most important factors to be elicited from the medical history. People with severe latex allergy can react with anaphylaxis as they enter a health-care facility where latex is present in the environment!
Chlorhexidine allergy
Chlorhexidine is a cationic chlorophenyl-biguanide antiseptic and disinfectant, commonly used as the diacetate or digluconate salt, and has excellent antimicrobial properties. Chlorhexidine allergy must be rare but is important, since chlorhexidine is present in many antiseptic preparations, including some mouth rinses, disinfectants and cosmetics. Medical devices that incorporate chlorhexidine include: intravenous catheters, topical dressings and implanted surgical mesh.
Chlorhexidine used topically, intra-urethrally as catheter lubricant, as a mouthwash and with impregnated catheters has been associated with hypersensitivity reactions and some deaths. Chlorhexidine has been used in the UK since 1954 and reactions have been reported since 1962, ranging from contact dermatitis, to urticaria to anaphylactic shock with cardiac arrest. Fatal cases of anaphylaxis prompted the FDA in 1998 to issue an alert to the medical community. Patients with suspected allergies should be investigated for evidence of chlorhexidine-specific IgE by skin or blood tests.
Management is along the lines for latex allergy (Ch. 17), essentially avoiding exposure, and using povidone-iodine as an alternate skin preparation or hexetidine mouthwash as an alternate for oral use.
Patch testing using chlorhexidine has revealed positive reactions in more than 2% of patients tested. In eczema patients, the rate may be as high as 5%.
Reactions to other dental materials
Dental materials such as alloys, bonding, cements, plastics, primers, resins and other materials can cause allergies leading to dermatitis, rashes, blistering, itching or burning – though these features are usually seen only in people highly sensitive to epoxy resins. Illustrative of the difference between skin and mucosa in response to sensitizing agents is the fact that a substance, to which the patient is sensitized when it is put in the mouth, can occasionally induce the typical skin rash. This has been reported, for example, in the case of nickel sensitivity when a nickel-containing denture caused a rash but no oral reaction.
Alloys
With few exceptions, metals used in dentistry are alloys – solid mixtures of two or more metals.
‘Gold allergies’
Gold alloys may occasionally be used for dental restorations or prostheses. Pure gold, because of its softness, is not indicated for use in the mouth except as gold foil – rarely used now. The basic alloys used are casting gold, gold solder, wrought gold and gold plate, and these may contain silver, copper, platinum, palladium or zinc. Lichenoid oral reactions to gold alloys are rare.
‘Mercury allergy’
Dental amalgam is a combination of mercury mainly with a silver–tin alloy. The American Dental Association (ADA) specifies that the alloy must include a minimum of 65% silver, and maximums of 29% tin, 6–13% copper and 2% zinc by weight. Alloys with high copper content usually have lower creep values than conventional silver–tin alloys. Some alloys are completely zinc-free and can therefore be used more successfully in a moisture-contaminated environment. Dental amalgam may cause mucosal reactions such as lichenoid lesions, but the evidence is controversial and mercury absorbed from amalgams into the mucosa frequently causes no histological reaction.
Mercury and its salts are potential sensitizing agents; exposure to them can occasionally lead to contact dermatitis, and the frequency of positive patch tests increases as dental students progress through their course. Also, a few patients have genuine contact sensitivity to mercury, though they can generally tolerate the placement of amalgam restorations, provided that none is spilt on to the skin. ‘Baboon syndrome’ is a special form of systemic contact-type dermatitis manifesting with bright red buttocks and flexural eczema that occurs after ingestion or systemic absorption of a contact allergen in individuals previously sensitized by topical exposure to the same allergen in the same areas; it has been reported as a reaction to mercury.
Another group of patients appear to have symptoms such as headache, lassitude or a general feeling of ill-health that they ascribe to mercury toxicity, although there is no reliable evidence that mercury underlies these problems. Such patients may also suffer more frequently from other unrelated complaints, such as chronic craniofacial pain, than do controls.
Nickel allergy
Nickel-containing alloys are widely used, especially in orthodontics. The highest nickel content is in nickel–titanium, especially in flexible wires, but even stainless steel contains almost 10% nickel; most of this is bound, however, and unlikely to cause allergy – so can safely be used in nickel-allergic people. Nickel allergy often arises from jewellery and the prevalence appears to have increased with the advent of body piercing. Safe alternatives to nickel wires include stainless steel, composites, titanium and gold-plated wires. Wires of nickel–titanium that are ‘altered’ or coated with plastics or resin might be safe. Brackets of stainless steel, titanium, ceramics or polycarbonates are safe. Plastic-coated studs on headgear will avoid the issue with nickel studs.
‘Titanium allergy’
Degradation of metallic biomaterials may result in products leading to metal hypersensitivity reactions. Titanium has been considered an inert material but it has been suggested that it can rarely induce toxicity or allergic type I or IV reactions in susceptible patients. In a systematic review it has been shown that titanium allergy develops among patients at any age, the most common clinical manifestations being dermal inflammatory conditions and gingival swelling. A significantly higher risk of positive allergic reactions was found in patients showing allergic symptoms after implant placement or unexplained implant failures. The risk of an allergy to titanium is increased in patients who are allergic to other metals. Alternative materials (e.g. zirconium oxide) are available.
Cements
Dental cements include zinc phosphate, zinc oxide and eugenol, polycarboxylate (zinc oxide powder mixed with polyacrylic acid) and glass ionomer cements (GICs). Allergic reactions to most dental cements are rare but GICs contain a polyalkenoic acid such as polyacrylic acid plus a fluoride-containing silicate glass (fluoroaluminosilicate) powder, and do occasionally cause reactions. Resin-modified glass ionomer cements (RMGICs) usually contain HEMA (hydrophilic monomer) plus a fluoride-containing glass and polyacrylic acid. Tri-cure GICs also incorporate a chemical curing tertiary amine-peroxide reaction to polymerize the methacrylate, along with the photo-initiation and acid–base ionic reaction. These resins may cause reactions.
Plastics and resins
Some chemicals in resins may cause reactions. Polymethyl methacrylate (PMMA), polystyrene, polyvinylchloride (PVC) and so on contain potential allergens such as long hydrocarbon-based chains produced from small monomer units (e.g. methyl methacrylate [MMA], styrene, vinyl chloride, etc.). A few individuals may react to the potential allergens shown in Box 29.1 and some reactive materials may leach from set material at least for a few days, as may degradation products (e.g. formaldehyde).
PMMA (acrylic) is used for clothing, plastic items or dentures and is itself probably inert, but dental MMA contains a wide range of components that are potentially sensitizing (Table 29.1). There are rare cases, particularly in dental health-care professionals (HCPs), of allergic reactions to MMA monomer and associated chemicals. Affected persons should avoid exposure. Surgical rubber, latex or vinyl gloves offer little, if any, protection, as they are quickly penetrated by the chemicals, but commercial laminated disposable protective gloves are available.
Table 29.1
Typical components of dental methyl methacrylate
| Liquid | Powder |
| Dimethacrylate (cross-linker) | Organic peroxides (initiators) |
| Hydroquinone (inhibitor) | Pigments |
| Methyl methacrylate (monomer) | Polymethyl methacrylate |
| Organic amines (accelerator) | Titanium dioxide |
| Ultraviolet absorber |
Resins are also used in dentistry as bonding agents, composite resins, pit and fissure sealants, and resin-based cements. Often referred to as direct-filling resins, they are of several types; the older type was an unfilled polymethacrylate, while the more recent type is a composite resin based mainly on dimethacrylates plus silanated glass. Acid-modified composites (compomers) consist of methacrylate monomers such as urethane dimethacrylate (UDMA) and fluoride-containing glass. The monomers most commonly used are UDMA and 2,2,-bis(4(2-hydroxyl-3-methacryloxyloxypropoxy)-phenyl) propane – bisphenol A glycidal methacrylate (BPA; BIS-GMA) – combined with lower-molecular-weight monomers such as triethylene glycol dimethacrylate (TEGDMA). Photo-initiators such as camphoroquinone facilitate polymerization via light curing.
The major sensitizers appear to be EGDMA, 2-HEMA, 2-hydroxypropyl methacrylate (2-HPMA), MMA and acrylated epoxy oligomer. 1,4-Butanediol dimethacrylate (BUDMA) and UDMA seem to be only weak sensitizers.
Allergic reactions in the mouth are typically lichenoid but occasionally urticarial or anaphylactoid, and dermatological reactions in HCPs are usually contact dermatitis or eczema – so direct skin contact is best avoided. Allergic reactions may be produced by the catalyst methyldichlorobenzene sulphonate in dibenzyltoluol present in Impregum and by the catalyst methyl p-toluene sulphonate in dibenzyltoluol present in Scutan. Some people are sensitive to colophony resins, found in wound sticking plasters, periodontal dressings, impression materials, cements, fix adhesives and some fluoride varnishes (e.g. Duraphat). Eugenol in periodontal dressings can also be a contact allergen.
Apart from allergic reactions, there are few data to indicate significant toxicity from most resin components, though BPA may have some oestrogenic activity in vitro. BPA has also been implicated in heart disease and diabetes, though only minuscule amounts are found in dental resin restorations, and much more is present in other items such as plastic bottles and can linings. BPA leaches out from food and beverage containers, as well as from dental resins. Indeed, a recent survey by the Centers for Disease Control and Prevention (CDC) found bisphenol in the urine of 95% of people in USA. High levels of BPA have been correlated with obesity, diabetes, cardiovascular diseases, polycystic ovaries or low sperm count. In 2008, the ADA stated:
There is also evidence that some dental sealants, and to a lesser extent dental composites, may contribute to very low-level BPA exposure. The ADA fully supports continued research into the safety of BPA but, based on current evidence, the ADA does not believe there is a basis for health concerns relative to BPA exposure from any dental material.
Concerns About Denture Fixatives
Some denture fixatives contain zinc, which may be absorbed and cause copper deficiency, myelopathy and ‘human swayback disease’ with polyneuropathies.
Concerns About Fluoride
Some studies, now criticized for their analyses, suggested associations of bladder cancer with occupational fluoride exposure and of osteosarcoma with water fluoridation. A recent systematic review confirmed the benefit of fluoride in protection against dental caries, and found that water fluoride to a concentration of 1 ppm had no proven adverse effects on bone strength, bone mineral density, fracture incidence (protective or deleterious), cancer incidence or mortality, or on other conditions such as prevalence of Down syndrome, congenital abnormalities or stillbirths. For details, see http://www.nhmrc.gov.au/guidelines/publications/eh41 (accessed 30 September 3013).
Another recent systematic review and meta-analysis, however, produced results that supported the possibility of an adverse effect of high fluoride exposure on child neurodevelopment.
Concerns About Mercury
Mercury is a well-known neurotoxin and nephrotoxin. There also may be an association between mercury exposures over time and coronary artery disease, including myocardial infarction. There are three kinds of mercury involved in exposure (and poisoning): organomercury, inorganic mercury and elemental (metallic) mercury poisoning.
Organic mercury poisoning from methylmercury is the most common type; it is well recognized and, except in rare cases (e.g. exposure to laboratory reagents), is exclusively associated with fish ingestion. Mercury in the environment, especially that released into the air through industrial pollution, falls into and can accumulate in rivers and oceans, where it is turned into methylmercury. Fish absorb the methylmercury as they feed and so it accumulates in them. Shark, swordfish, king mackerel and tilefish contain high mercury levels, and white tuna can have fairly high levels. The most commonly eaten fish that are low in mercury are shrimp, canned light tuna, salmon, pollock and catfish. The US Food and Drug Administration (FDA) outlines pregnancy concerns and fish mercury levels at http://www.fda.gov/Food/FoodborneIllnessContaminants/Metals/default.htm (accessed 30 September 2013).
The most infamous example of methylmercury poisoning was at Minamata Bay and Nigata, Japan, in the 1950s. Many people suffered neurological damage, some died and there was a high incidence of cerebral palsy in neonates (Minamata disease) as a result of eating fish contaminated by mercury in industrial discharge found in the sea. Mercury poisoning of this type continues as a problem in parts of China, Brazil, Peru, Philippines, Suriname and Venezuela, and is associated mainly with occupations such as mining activities. Examples of gold mining-associated mercury pollution (often with cyanide and arsenic) are known in Africa, Canada, China, Philippines, Siberia, South America and USA. For further information, see http://www.nrdc.org/health/effects/mercury/medical.asp (accessed 30 September 2013).
Inorganic mercury salts poisoning, now rare, caused acrodynia (pink disease), a condition seen mainly at the end of the nineteenth century in children and others who used calomel (a mercury salt) in teething powders and gastrointestinal medications; it was also caused by inorganic mercurials used as disinfectants in detergents and baby powders. Inorganic mercury poisoning is still occasionally seen in people using mercuric chloride in skin creams available in the developing world, in some traditional Chinese and herbal preparations, in some ritual spiritual or ‘medical’ practices, in suicide attempts and in forensic science, as well as in bizarre accidents. Poisoning can cause:
■ acrodynia – skin peeling, salivation, hypotonia, swelling of extremities
Metallic mercury poisoning is an issue with dental amalgam but is actually confined almost exclusively to the occupational setting. Dental amalgam is a mixture of 50% metallic mercury with other metals, and there is concern that mercury can be released as vapour, ions or fine particles, which can be inhaled or ingested. Studies of amalgam exposure, even during pregnancy, have not documented any toxicity, including birth defects, neurologic sequelae, spontaneous abortions or reduction in fertility. Poisoning was, however, well recognized in the past among people working with metallic mercury (e.g. thermometer makers and makers of felt hats – hence, ‘mad as a hatter’). Poisoning has more recently been reported among people who use mercury for activities such as gold mining, where mercury is added to the pan to amalgamate the gold and is subsequently boiled off. Metallic mercury is also used in Santería – a Caribbean religious practice in which mercury may be sprinkled inside a home and can cause serious over-exposure. Accidents also continue to occur, metallic mercury still being found in some older equipment such as thermometers, fluorescent lights and computers. Mercury can be absorbed by inhalation of its vapour or through skin and mucous membranes (and mercury salts can be absorbed after their ingestion). There are even some who inject it intravenously in suicide attempts! Mercury is highly lipid-soluble, rapidly penetrates the blood–brain barrier and infiltrates neurons.
Acute poisoning by massive inhalation of mercury vapour is rare but can cause potentially fatal pneumonitis and neurological symptoms, particularly tremor and excitability. Chronic poisoning by mercury vapour inhalation primarily causes lassitude, gastrointestinal disturbances, anorexia and weight loss, and affects the central nervous system (CNS) with tremor, memory loss and excitability (erethism). Other effects include hypersalivation, accelerated periodontitis and a black gingival line due to deposition of mercury sulphides (like the lead line). Rarely, jaw necrosis follows.
Mercury hazards to dental health workers
Amalgam use may be associated with occupational exposure of dental health workers (DHWs) to metallic mercury if personnel fail to follow good work practices. On average, up to 1.5 kg of mercury are used by a dental practice annually; in the past, when less attention was paid to occupational hazards, mercury vapour in the surgery atmosphere and its levels in the blood, hair, nails and urine of DHWs were frequently above controls. Droplets of mercury could also accumulate in significant amounts in surgery carpeting or crevices in the floor. Mercury is also absorbed during the outmoded practice of hand trituration of amalgam or during other skin contact. In the past, after decades of practice, a few DHWs suffered chronic mercury toxicity with tremor, incoordination, polyneuropathies and accelerated senility. Autopsy studies have also shown mercury deposits, particularly in the pituitary glands and occipital lobes. Rarely, deaths have been reported after prolonged heavy exposure.
In a study of female DHWs, a history of reproductive failures, menstrual disorders and spina bifida in their children was suggested to be related to mercury levels in hair, but this has not been widely confirmed – and other larger studies have shown no such correlation. Indeed, the perinatal death and birth defect rate for infants born to dentists is currently lower than average. Further, urinary mercury levels appeared to be falling in studies of dentists in the 1990s, and more recent studies of DHWs have not shown excessively high urinary mercury levels where good mercury hygiene was practised. Another study found no consistent association between either urinary mercury or chronic mercury exposure and any category of self-reported symptoms of depression, anxiety and memory loss. Exposure of DHWs to mercury is currently now very low – probably as a result of increased awareness of mercury toxicity and improved methods of preparing amalgam – especially the use of encapsulated mercury amalgam. Thus it seems unlikely that there is now a significant risk to DHWs who work in practices where adequate standards of mercury hygiene appertain. Indeed, mercury levels in DHWs are highest in those who eat fish, indicating that at least part of their mercury burden was from dietary – not dental – sources.
Risks from dental amalgam restorations
Dental amalgam restorative materials continually emit mercury vapour, which is increased by chewing, eating, brushing and drinking hot liquids. The World Health Organization’s maximum recommended mercury intake is 2 micrograms/kg/day; release of mercury from dental amalgam is around 10 micrograms/day. Hydrogen peroxide can increase mercury release, so should be avoided. Removal of old amalgams from teeth with an air-rotor produces traces of mercury vapour – but not if adequate water-cooling and aspiration are used. The responses of official bodies to public concerns about mercury toxicity are shown in Appendix 29.1, and suggest a very low risk of toxicity. In one large study, children who received dental restorative treatment with amalgam did not, on average, have statistically significant differences in neurobehavioural assessments or in nerve conduction velocity, compared with children who received resin composite materials only. In the New England Children’s Amalgam Trial, a randomized study involving children, the hypothesis that restorations using amalgam resulted in worse psychosocial outcomes than restorations using composite resin over a 5-year period following initial placement was not supported by the results. These findings, combined with the trend of higher treatment need later among those receiving composite restorations, suggested that amalgam should remain a viable dental restorative option for children.
Thus, according to present scientific evidence, the use of amalgam is not a proven health hazard and, to date, there is no dental material that can fully substitute for amalgam as a restorative material. More recent studies and reviews have also found little to no correlation between systemic or local diseases and amalgam restorations, although extensive amalgam restorations in pregnant or nursing women are not recommended. Nevertheless, the state governments of California, Connecticut, Maine and Vermont, and the federal governments of Denmark, Finland, Norway and Sweden have legislation requiring that dental patients receive informed consent information about the restorative material that will be used. Apart from Norway, Denmark and Sweden, dental amalgam has not been banned in any European Union country. A European Commission report by the BIO Intelligence Service (BIOIS), however, recommends the phasing-out of amalgam, and of mercury in button cell batteries (http://ec.europa.eu/environment/chemicals/mercury/pdf/review_mercury_strategy2010.pdf; accessed 30 September 2013).
Mercury disposal
The greatest hazard from inhalation of mercury vapour is as a result of any spillage. Dentists should use dental amalgam separators to trap excess amalgam waste coming from surgeries and avoid release into the sewers; separators generally have a removal efficiency of approximately 95%. Captured amalgam solid waste should be appropriately recycled or retorted. Amalgam in waste waters proceeds to publicly owned treatment works (POTWs), most of which have more than 90% efficiency at removing amalgam waste. The rest is discharged from POTWs as a component of sewage sludge, which may then be disposed of:
■ in landfills – where mercury may be released into the groundwater or air
■ through incineration – when mercury may be emitted into the air
Mercury waste from dental surgeries thus contributes significantly to the overall mercury contamination in wastewater, and may be the source of about 50% of all mercury entering POTWs – far exceeding pollution from all other commercial and residential sources. Other amalgam constituents (e.g. silver, tin, copper and zinc) have not been reported in dental clinics’ wastewater.
Cremation and burial can also be responsible for mercury pollution. Cremation has recently gained in popularity due to the shedding of negative religious connotations but it releases quantities of atmospheric mercury from amalgams. Burial is important for many cultures, such as Catholics and Muslims; it also releases mercury and might reach drinking water sources. This issue is being addressed (http://www.epa.gov/hg/dentalamalgam.html and http://ec.europa.eu/environment/chemicals/mercury/pdf/review_mercury_strategy2010.pdf; both accessed 30 September 2013). The United Nations Environmental Programme (UNEP) has expressed concerns and also outlined the action required (http://www.unep.org/hazardoussubstances/mercury/tabid/434/default.aspx; accessed 30 September 2013).
Concerns About Tooth-Whitening Products
Tooth-whitening products are widely available for sale over the counter (OTC) or ARE dispensed by dentists for patients’ use at home. Most products release hydrogen peroxide (H2O2). Self-applied bleaching agents either contain or generate H2O2, as gel in trays, paint-on films or whitening strips. A nightguard bleach typically uses 10% carbamide peroxide (which contains approximately 3% H2O2) and OTC whitening strips contain approximately 6% H2O2, while in-surgery bleach uses 25–35% H2O2. These can all potentially produce comparable whitening but strips are slightly more effective at whitening than is a gel in a tray. In the UK, Cosmetic Product (Safety) Regulations apply to products that contain or release more than 0.1% H2O2.
Recently, attention has focused on dentist-prescribed home bleaching, in which the teeth and oral soft tissues can be in contact with peroxide-type agents for long periods; this creates a different situation from the use of in-surgery bleaching or home oral health-care products, such as peroxide-containing toothpastes and mouthwashes, from the standpoint of both dose and time. Hydrogen peroxide is a highly reactive substance that can damage oral soft and hard tissues when employed at high concentrations and with prolonged exposures. Direct exposure of mucosae, skin or eyes to 30% H2O2 may cause severe irritation, burns, blistering and ulceration. The much lower concentrations used in dental whitening products do not appear to produce such adverse effects, but tooth sensitivity and gingival irritation are common.
Concerns About Oral Health-Care Products
Contact dermatitis can occasionally be precipitated on the lip vermilion or, more frequently, the perioral skin by components of toothpastes, lipsticks and some foods (notably mangoes and oranges) or by dental impression materials. Toothpastes or chewing gum components, especially cinnamon, can also cause plasma cell gingivitis or inflammation of other parts of the oral mucosa. Gingivitis, cheilitis, perioral dermatitis and other lesions have been described in patients using tartar-control toothpastes that contain cinnamonaldehyde or pyrophosphates. Some substances (e.g. zirconium) occasionally cause granulomatous oral reactions, and allergic reactions may underlie orofacial granulomatosis.
Drugs
Relatively few drugs are used in general dentistry, and the rarity of hypersensitivity reactions and lack of evidence of significant interactions of local anaesthesia (LA) with drugs must be stressed. The many relative and absolute drug contraindications, as well as drug interactions, are discussed in Chapter 3 and its appendices. Drugs used in the practice of oral medicine and surgery may also produce adverse reactions or interactions.
However, children need lower doses of virtually all drugs. Older people tend to be more liable to adverse drug reactions, and the reactions tend to be more serious and last longer than in younger people. Older people are also more likely to be given drugs.
Sedative techniques are more likely and general anaesthesia (GA) is much more likely to produce adverse reactions.
Drug Reactions and Allergies
Any suggestion of a previous drug adverse reaction or allergy, and particularly any adverse reaction during anaesthesia or imaging, must be taken seriously. Drug allergy or hypersensitivity results from interactions between a drug and the immune system, mediated by immunoglobulin E (IgE), but some reactions involve additional, poorly understood mechanisms. Identifiable risk factors for drug reactions include:
Furthermore, patients with allergy to one drug, and individuals with Sjögren syndrome or human immunodeficiency virus (HIV) disease may be particularly liable to drug allergies.
Drug hypersensitivity is usually a clinical diagnosis made on the basis of a rash or anaphylaxis. Laboratory testing may be useful, with skin testing providing the greatest specificity. Treatment includes discontinuation of the offending agent, symptomatic treatment and patient education.
Drug-induced hypersensitivity syndrome (DIHS) is a glandular fever-like syndrome (fever, rash, cervical lymphadenopathy, raised white cell count with atypical lymphocytes, and liver dysfunction) that follows the use of certain drugs – especially anticonvulsants such as carbamazepine and phenytoin, isoniazid and sulphonamides. Many cases also appear to be associated with the reactivation of human herpesvirus 6 (HHV-6) or other herpesviruses.
Many drug reactions could not be predicted and it is only by recording suspected adverse drug reactions to an appropriate authority (Figs 29.1 and 29.2) that serious adverse reactions of apparently safe drugs can be elicited (e.g. the cardiotoxicity of cyclo-oxygenase-2 inhibitors). Drug interactions are possible and it is also important to bear in mind that OTC and herbal preparations, foods and other substances can sometimes interact by affecting drug absorption or efficacy, or by causing other interactions (Ch. 27 and Appendix 3.3).
Drug Adverse Effects
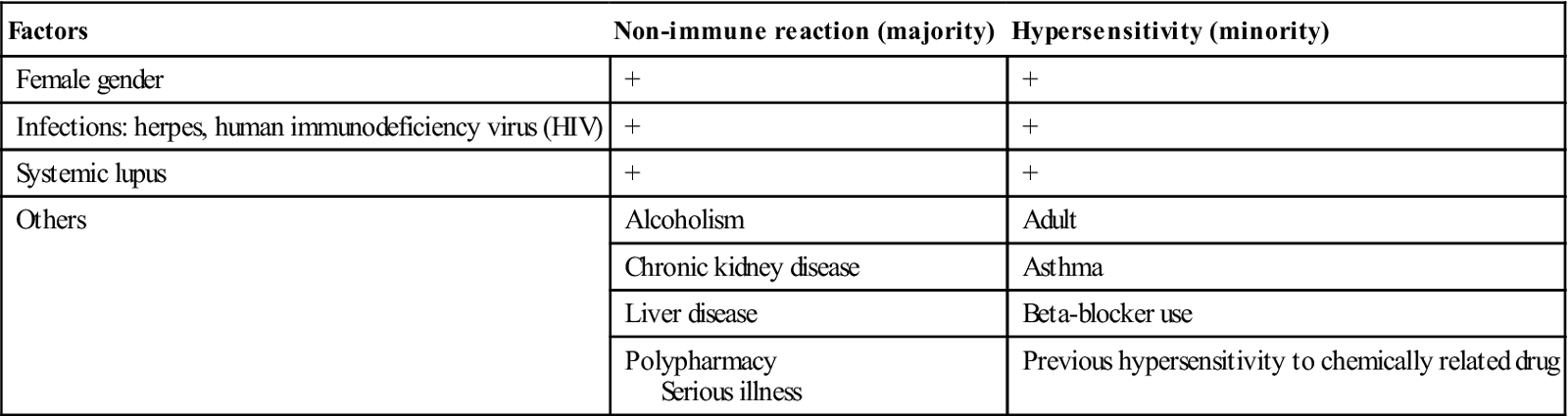
Many adverse drug reactions are probably not, at present, recognized. A full medical history should always be taken, asking specifically about adverse drug reactions. Certain conditions increase the risk of adverse reactions (Table 29.2) and can influence the choice of drugs. The excessive use of virtually any drug can cause harm. Patients should be warned if serious adverse reactions are likely, and provided with the appropriate warning card.
Table 29.2
Risk factors for adverse drug reactionsa
< ?comst?>
| Factors | Non-immune reaction (majority) | Hypersensitivity (minority) |
| Female gender | + | + |
| Infections: herpes, human immunodeficiency virus (HIV) | + | + |
| Systemic lupus | + | + |
| Others | Alcoholism | Adult |
| Chronic kidney disease | Asthma | |
| Liver disease | Beta-blocker use | |
| Polypharmacy Serious illness |
Previous hypersensitivity to chemically related drug |
< ?comen?>< ?comst1?>
< ?comst1?>
< ?comen1?>
Anaesthetics and related agents
The use of excessive amounts of LA agents can be dangerous. Lidocaine with adrenaline (epinephrine), the most widely used drugs in dentistry, have proved to be remarkably safe in practice over very many years but gross overdose can be dangerous. One dentist has been found guilty of manslaughter, having given 16 cartridges of lidocaine with adrenaline to an elderly patient, who subsequently died.
Intravenous anaesthetic agents can cause anaphylactic-type reactions, either because of hypersensitivity or by directly inducing histamine release (the term ‘anaphylactoid’ may then be more appropriate). Tachycardia, vascular collapse and skin reactions (flushing, oedema or urticaria) are the most frequent signs, but reactions can range from minor symptoms to bronchospasm and a sharp fall in blood pressure. Treatment is as for anaphylaxis.
Anaphylactoid reactions have been reported particularly for thiopental. Such reactions have sometimes been fatal, probably because of failure to recognize their nature, since the loss of consciousness that results must be distinguished from the onset of anaesthesia; many intravenous agents have been discontinued because of this.
Suxamethonium, the muscle relaxant, may also cause an anaphylactoid reaction but alcuronium, tubocurarine and other relaxants are sometimes implicated; as there does not need to be previous exposure to the agent, cross-reacting antigens are presumably implicated.
Halothane hypersensitivity may be a cause of ‘halothane hepatitis’ (Ch. 9). LA agents have been routinely used since the late nineteenth century but adverse reactions and allergy are not common (Ch. 3). True IgE-mediated LA allergy must be very low indeed; one recent paper reviewed 23 case series involving 2978 patients with reactions and found only 29 patients with true IgE-mediated allergy to LA (less than 1%).
Hypersensitivity to the ester-type LA agents, which used to be commonly employed, is low and esters such as amethocaine and benzocaine are now restricted mainly to use in surface anaesthetics. The risk with amide LAs, such as articaine, lidocaine, mepivacaine or prilocaine, is probably lower. Lidocaine rarely causes reactions. The most sensitizing component of LA solutions has been the preservative: initially, methylparabens was used (parabens are now rarely used). There may be allergies to sulphite antioxidants or to latex in the LA cartridge diaphragm or bung (Ch. 17).
Attempts to confirm putative allergy to LAs by skin testing (prick tests or intracutaneous tests) are time-consuming and usually uninformative; they can provoke anaphylaxis in the uncommon instances of true allergy and are therefore rarely justified. Challenge tests are also typically negative. Where there are multiple supposed allergies, it may be best to administer sterile saline subcutaneously first to demonstrate any non-allergic response, and then give rising concentrations of diluted LA at 30-minute intervals (resuscitation facilities must be at hand). Clearly, it is of great importance to avoid labelling patients inappropriately as allergic to LAs in the first place. If a patient claims to be allergic to an LA, then a non-cross-reacting agent should be used; alternatively, a GA may be required.
Analgesics
Non-steroidal anti-inflammatory drugs (NSAIDs) may, rarely, induce allergic reactions. Aspirin can provoke allergic reactions but, in relation to the scale of its use, these are almost negligible. Aspirin-induced asthma is a rare possibility, seen mainly in patients with nasal polyps (‘triad asthma’ – asthma, nasal polyps and aspirin sensitivity). Other NSAIDs may, even more rarely, induce allergic reactions. If there is a history of allergy to a drug, it should not be given.
NSAIDs should not be used in patients taking anticoagulants, alcohol or high-dose methotrexate. They should also be avoided in older or renally impaired patients on digoxin, and avoided over the long term in those taking other NSAIDs. It is possible, but unconfirmed, that NSAIDs should not be given to patients taking lithium. NSAIDs are probably appropriate in the short term for patients taking antihypertensives, unless they have severe congestive heart disease or renal function is compromised.
Aspirin is a safe analgesic but readily causes platelet dysfunction, and excessive doses have led to post-extraction bleeding. It should also not be given to patients taking oral hypoglycaemics, valproic acid or carbonic anhydrase inhibitors.
Paracetamol (acetaminophen) is also safe but, when given in repeated doses, can cause severe liver damage. It may be used in the short term for any patient with a healthy liver, but should not be given to heavy drinkers or to persons who have recently stopped alcohol after chronic intake.
Opioids should not be given to heavy alcohol drinkers.
Antibiotics
Some antimicrobials (erythromycin, clarithromycin, metronidazole, ketoconazole and itraconazole) give rise to potentially life-threatening interactions with a host of other drugs, whose metabolism is impaired by them.
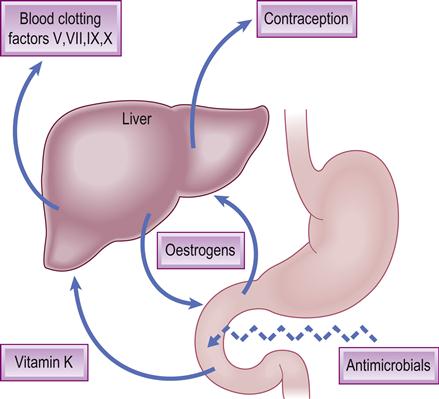
Antibiotics may also interfere with the effects of warfarin via suppression of gut bacteria that produce vitamin K. Commonly employed antibiotics may also impair the effectiveness of oral contraceptive agents, though the evidence for this is not strong (Fig. 29.3).
Anaphylaxis to antibiotics is a real possibility and has been estimated to have caused approximately 300 deaths annually in USA. Hypersensitivity reactions to beta-lactams (in penicillin and cephalosporins) are the most common reactions and are more likely to follow parenteral than oral administration. Patients allergic to penicillin usually react to any other penicillin except aztreonam. Individuals with penicillin allergy should avoid carbapenems and use caution with cephalosporins. About 10% of those sensitized to penicillin are said also to react to the cephalosporins. However, some allergic reactions can be selective for certain semi-synthetic penicillins, and some patients tolerant of benzylpenicillin can show delayed reactions to aminopenicillins. The causal antigen appears to be the penicilloyl group, which forms from metabolic cleavage of the beta-lactam ring and acts as a hapten, binding to body proteins to become antigenic. Specific IgE antibodies to penicillin are the cause of the anaphylactic reactions by binding to mast cells and triggering mediator release. There appears to be no association between penicillin anaphylaxis and atopic disease, despite both being mediated by the same mechanism. The most common drug allergic reactions, apart from anaphylaxis, are urticarial and irritating rashes or sometimes a serum sickness type of reaction (joint pains and fever follow days or weeks after administration).
A history of previous reactions to penicillin suggests a greater risk of acute anaphylaxis – but there is no completely reliable method of prediction. Unfortunately, the first manifestation of sensitivity can occasionally be anaphylaxis and, rarely, a patient who has had penicillins on several occasions without ill effect can suddenly develop anaphylaxis. A negative history therefore reduces the chances but does not exclude the possibility of anaphylaxis when the patient claims to be allergic to penicillin. From the practical and medicolegal viewpoints, therefore, reliance has to be placed on the history and an alternative antimicrobial should be used – but never any penicillin derivatives (except aztreonam). Since a negative history of penicillin allergy does not totally exclude the possibility of anaphylaxis, it is arguable that penicillin should not be given immediately before a GA, since recognition under such circumstances can be difficult. If penicillin has to be given, 30 minutes should be allowed to elapse before induction of GA; after such a period, a severe reaction is unlikely. Depot penicillins, which are slowly excreted, can maintain the antigenic challenge so that treatment of an allergic reaction may have to be continued until all the antigen is used up. Occasionally, procaine penicillin can additionally cause vertigo, hallucinations and acute anxiety reactions if given intravenously (or if it accidentally enters a vein) – reactions that may be mistaken for anaphylaxis.
A patient should, of course, be lying down when any injections are given, as fainting after injections is common and may be confused with anaphylaxis.
Anticonvulsants
Anticonvulsants are used to treat epilepsy and trigeminal neuralgia. Anticonvulsant hypersensitivity syndrome (AHS) is a potentially fatal, drug-induced, multiorgan syndrome reported with carbamazepine, phenytoin, phenobarbital and lamotrigine. The exact mechanism of AHS is unclear but it may have three components: deficiency or abnormality of the epoxide hydroxylase enzyme that detoxifies metabolites of aromatic amine anticonvulsants; reactivation of herpesviruses; and ethnic predisposition, especially an association with human leukocyte antigen (HLA)-B*1502 (HLA BFNx011502 allele) in some Asian but not in Caucasian and Japanese patients.
Whilst up to 1 in 5 patients on phenytoin may develop skin eruptions, only a small proportion will progress to AHS – with urticaria, purpura, erythema multiforme and exfoliative dermatitis. Toxic epidermal necrolysis is uncommon, and usually occurs in patients who are re-exposed or who continue to receive anticonvulsants after hypersensitivity has developed. AHS is a clinical diagnosis; fever, rash and hepatitis are common features. Treatment is symptomatic. The offending drug should be immediately discontinued. Topical steroids and antihistamines are helpful in controlling symptoms. Systemic corticosteroids are often used.
Radiocontrast media (RCM) reactions
Intravascular iodinated RCM agents are based on a tri-iodinated benzene ring and can cause adverse reactions. These can be:
RCM usually act by directly releasing histamine from mast cells rather than provoking an allergic response. High-osmolar contrast media (HOCM) are the oldest but have largely been superseded by less toxic non-ionic compounds – low-osmolar contrast media (LOCM). Additional modifications to reduce toxicity further include: adding calcium ions (reduces cardiotoxicity), having a neutral pH (low pH predisposes to vasodilatation), and altering number and distribution of OH ions (decreases neurotoxicity).
People at higher risk for reactions to RCM include those who:
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses