26 Other Uses of Fluoride in Caries Prevention
Fluoride (F) was first used to prevent dental caries by adding it to drinking water, but it was not long before alternative methods were put into practice. The result today is that F is used in a variety of ways to control caries in public health programs, individual patient care, and self-care. Dental professionals today must use F with the knowledge that there is extensive background exposure to F through drinking water, toothpaste, mouthrinses, and processed food and drink. We stated in Chapter 24 that F works best to control caries when low ambient levels are constantly present in the oral cavity (in plaque, saliva, enamel surface, soft tissues). Any use of F rinses, professionally applied gels, or F dietary supplements should be aimed toward achieving that condition, either for the individual patient or for the public.
FLUORIDATED SALT
F salt was first used in Switzerland in 1955. There is no water fluoridation in Switzerland, and the nation has only one salt processing and distribution company. Switzerland is a prosperous, highly developed country, and there have been few problems with safety and control of the procedure. The reduction in caries incidence in Swiss communities in which F salt has a good market share, although measured only in before-after designs, appears similar to that found with water fluoridation.38,110,146 F salt has been well accepted by the Swiss public and now accounts for approximately 80% of the salt market. The effects of salt fluoridation have also been studied in Colombia65 and Hungary150 with generally favorable results, and good results have been reported in Jamaica58,112 and Mexico.81 Worldwide, it is estimated that approximately 40 million people consume F salt daily.125
Although the evidence for the effectiveness of F salt is consistent, it comes from only a limited number of observational studies rather than from clinical trials. The results of the observational studies have been contaminated by the concurrent use of F toothpaste, so that not all of the observed reduction in caries can be attributed to consumption of F salt.165 In terms of how F salt works to inhibit caries, salivary F concentration has been found to show a small but significant increase for at least 5 minutes after ingestion of the F salt, and these levels return to baseline in 20 minutes.103 Given the role of salivary F as a reservoir (see Chapter 24), this appears to be how F salt produces its beneficial effects.
The concentration of F in salt is based on estimates of salt consumption and is evaluated by studies of urinary F concentration. The material most commonly used to fluoridate salt is potassium fluoride (KF), although sodium fluoride (NaF) is also used. The early Swiss studies began with salt fluoridated to 90 parts per million (ppm), but it soon became clear that this level was too low. In the early 1970s the concentration was increased to 250 mg KF/kg salt (or 225 mg NaF/kg salt), a level based on estimated adult salt consumption of some 8-10 g/day. Although patterns of salt consumption vary from one country to another,80 250 or 350 mg KF/kg salt now appears to be the standard in Switzerland and other countries as well.
FLUORIDATED SCHOOL DRINKING WATER
In rural areas in which community water fluoridation is not possible, the approach of fluoridating the schools’ drinking water was promoted for some years. The procedure was reported to reduce dental caries among schoolchildren by about 40%,75 although none of these studies was conducted blind and there were no concurrent controls.
Relative to community water fluoridation, the disadvantages of school water fluoridation are that children do not receive the benefits until they are old enough to begin school, and of course they drink the water only when school is in session. To compensate for this reduced exposure, the recommended concentration is 4.5 times the optimum for community-wide water fluoridation. At its peak, school water fluoridation was introduced in 13 states in the United States. Data reported by states to the Division of Oral Health of the Centers for Disease Control and Prevention (CDC) show that in 1981 school water fluoridation was established in 470 schools serving some 170,000 children. Its current extent is not known but is much lower than the 1981 peak. There is no record of the procedure’s being used in countries outside the United States.
Despite the CDC’s issuance of safety guidelines, a number of overspill mishaps have occurred, fortunately without lasting ill effects.3,72,163 The CDC no longer promotes school water fluoridation.
FLUORIDATED MILK
The mode of action seems to be from salivary return of F to the oral cavity.160 Salivary F levels are raised 45 minutes after ingestion,26 and plaque F levels are raised threefold over resting levels for up to 4 hours after ingestion.57,126 Urinary F levels in children are similar to those found in a community with water fluoridated at 1 ppm.88
Only one randomized double-blind trial has been conducted to test the efficacy of consumption of F milk,147 although a test of F milk readily fits the randomized design. Other studies examining the efficacy of milk fluoridation have been seriously flawed. A few public health programs using fluoridated milk have become established, such as in Bulgaria119 and in St. Helens, near Liverpool.97 However, it is hard to recommend further research into milk fluoridation for the United States in view of the large number of F vehicles available today.
DIETARY FLUORIDE SUPPLEMENTS
Caries Prevention by Fluoride Supplementation
Early studies of the preeruptive caries-preventive effects of F supplementation in children were often seriously deficient and thus yielded questionable results. Some of the highest caries reductions, around 80% over several years, were reported by American studies in the mid-1970s,1,105 but flaws in these studies included self-selection into test and control groups or the absence of concurrent controls, high attrition rates, and nonblinded examiners. The association that practitioners have observed between conscientious use of F supplements and freedom from caries also cannot be taken as evidence of efficacy, because compliance with the F supplementation regimen is naturally higher among dentally aware people who also have other good oral health habits.
Evidence to favor a preeruptive benefit from F supplementation remains limited to retrospective analyses. Positive results have been reported retrospectively,5,32,40,41,60,108,166,167 but self-selection bias was evident in all of these studies. Other retrospective studies found no difference in caries experience between those children who reported using F supplements and those who did not.14,17,64,74,86,155
Although the evidence for preeruptive benefits from F supplementation is weak, well-conducted randomized clinical trials using placebos and blinded examiners have shown that it can have posteruptive benefits in school-age children. Studies in which the supplements were chewed, swished, and swallowed under supervision have reported caries reductions of 20%-28% over 3-6 years.43,50 Caries reductions of 81% were reported in a Glasgow study in which children from lower socioeconomic groups who were initially 5.5 years of age sucked a 1-mg F tablet or a placebo under supervision in schools every school day for 3 years.148
Dosing Schedules
To obtain F supplements in the United States and Canada, a prescription by a dentist or physician is required; both the American Dental Association (ADA) and the American Academy of Pediatrics (AAP) maintain schedules of recommended doses of F supplements. However, before 1979 the two schedules did not coincide: the AAP first recommended 0.5 mg/day6 for children under 2 years of age, whereas the ADA recommended 0.25 mg/day. The AAP altered its earlier recommendations7 in 1979 to bring them into line with those of the ADA and reaffirmed these new recommendations in 1986.8
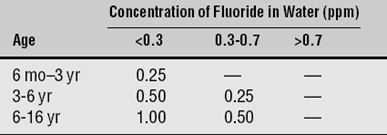
As the dosing schedules for F supplements are revised periodically, the trend has been to make them ever more conservative. In response to the growing evidence that use of F supplements is a risk factor for fluorosis, the ADA revised its schedule in 1994 to reduce the amount of F ingested by young children, and the AAP accepted this schedule a year later.9 The current ADA-recommended schedule, based on the age of the child and the concentration of F in the water supply, is shown in Table 26-1. The thinking in Europe is similarly conservative: a European Community group recommended that a supplement of 0.5 mg F be used only for “at-risk” individuals from the age of 3 years onward. This group also declared that F supplementation had no place as a public health measure.34
Fluorosis Risk From Fluoride Supplementation
Reduction of the early AAP recommendation of 0.5 mg/day for children under 2 years of age was overdue, for this dosage probably led to a considerable degree of fluorosis. One study in the Boston area that used the old AAP schedule, for example, was successful in terms of caries reduction, but 67% of the children developed very mild or mild fluorosis.2 Excess F ingestion in infancy and early childhood through inappropriate prescription of F supplements is unfortunately more common than it should be. A 1980 national survey of pediatricians found that around 20% of them who practiced in fluoridated areas were prescribing F supplements for child patients who lived in the same communities,104 a clearly inappropriate action that increases the risk of dental fluorosis. Things were no better 15 years later.124 These studies have emphasized the need for continuing education of both physicians and dentists. If supplements are to be prescribed at all, they should certainly not be prescribed for patients who consume fluoridated water.
The major concern now associated with supplement use is that of dental fluorosis. Although fluorosis can develop at any preeruptive stage, late secretion and early maturation have been identified as the developmental times when dental enamel is especially sensitive to ingested F.42,59,95 Although some studies found no association between supplement use and the development of fluorosis,15,171 considerably more have reported a clear association.2,39–41, 74,86,94,96,133,153,155,169,170 A series of excellent case-control studies has provided strong evidence for a cause-and-effect relation between use of F supplements and dental fluorosis,121–123 and a comprehensive meta-analysis also concluded that use of F supplements is a risk factor for fluorosis.83
Appropriate Use of Fluoride Supplements
Although F supplements are reportedly used by some 16% of American children under 2 years of age,117 their continued prescription in North America needs to be thoughtfully reviewed. As often is the case in public health, their use requires trade-off decisions: F supplements have some beneficial impact on oral health, but there is also the hazard of fluorosis when they are used in an era of wide exposure to F. The reasons why prescription of F supplements for infants and young children needs to be considered carefully can be spelled out as follows:
In light of the advantages and disadvantages of F supplements in a time of widespread F exposure, calls have been made for a reevaluation of their use among young children.14,82,132 They have a place when used to achieve a posteruptive effect in children older than 7 years, and it is likely that they would be useful in the growing population of older dentate people who are at risk of both coronal and root caries, although this issue has not yet been studied. At the very least, when F supplements are prescribed for infants and young children, parents or guardians should be informed of the fluorosis risk that accompanies the limited cariostatic benefits.
Prenatal Fluoride Supplementation
The question of whether to prescribe F supplements for an expectant woman to increase caries resistance in the offspring has been debated for years. In light of the discussion on dietary F supplements in general, it is not surprising that current views are that any enhanced resistance to caries will be only minor at best. The only prospective randomized trial of prenatal F supplementation found no significant difference in the caries experience of the offspring.100 Therefore use of prenatal supplements is not recommended.
F can cross the placental barrier and enter the fetal circulation.31,120 Fetal plasma F levels are correlated with maternal levels, although generally they are lower. F is taken up in the mineralizing tissues of the fetus; fetal enamel levels go up with an increase in maternal plasma F levels.120 The most critical time for F uptake in enamel, as noted earlier, is the late secretion and early maturation phase. As a result, prenatal F exposure will have little effect, especially since mineralization of even primary teeth is not far advanced at birth.
Collectively the research evidence suggests that prenatal F administration cannot be supported. As long ago as 1966, the U.S. Food and Drug Administration banned advertisements which claimed that prenatal F administration would increase the caries resistance of offspring.161 No convincing research has emerged since then to change that picture, and hence the ban still pertains. Although there is nothing to suggest that prenatal F supplementation will harm either fetus or mother, no evidence exists to support claims of prenatal benefit.
PROFESSIONALLY APPLIED FLUORIDE GELS
The F compounds that dental professionals routinely use in tray applications are highly concentrated, and careful attention to technique and to the amounts used is required. Table 26-2 lists the quantities and concentrations of the F compounds most frequently used in dental practice, as well as those used in public health programs and self-applied by individuals. Box 26-1 is a guide to estimating the amounts of F in dental products, and Box 26-2 brings together the information on toxic exposure to F.
Table 26-2 Concentration and quantity of fluoride in commonly used topical fluoride compounds
| Compound | Concentration (ppm) | Quantity |
|---|---|---|
| Topically applied agents | ||
| 2% NaF | 9050 | 45 mg in 5 ml* |
| 1.23% APF solution, gel, or prophylactic paste | 12,300 | 62 mg in 5 g |
| 8% SnF2 solution | 19,363 | 97 mg in 5 ml |
| 0.4% SnF2 gel | 968 | 4.9 mg in 5 g |
| 5% NaF varnish (2.26% F) | 22,600 | 51 mg in 5 ml |
| Mouthrinses | ||
| 0.2% NaF—weekly | 905 | 9 mg in 10 ml† |
| 0.05% NaF—daily | 226 | 2 mg in 10 ml |
| 0.1% SnF—daily | 242 | 2 mg in 10 ml |
| APF rinse (0.1% fluoride)—weekly | 1000 | 10 mg in 10 ml |
| APF rinse (0.022% fluoride)—daily | 200 | 2 mg in 10 ml |
| 1 mg in 5 ml of oral rinse supplement (rinse and swallow) | ||
| Toothpastes | ||
| 0.76% Na2 FPO3 | 1000 | 1 mg/g‡ |
| 0.243% NaF | 1105 | 1.1 mg/g |
| 1.14% NaF | 1500 | 1.5 mg/g |
| 0.4% NaF | 966 | 1 mg/g |
| 0.4% Na2 FPO3 | 526 | 0.5 mg/g |
| 0.304% Na2 FPO3 | 401 | 0.4 mg/g |
Note: Some figures are rounded.
APF, Acidulated phosphate fluoride.
* A topical application or prophylactic treatment uses about 5 ml or 5 g of material.
† Amounts of 5 and 10 ml are used in supervised mouthrinsing.
‡ An average load of toothpaste on the brush is about 1 g.
BOX 26-1 How To Estimate the Amount of Fluoride in a Dental Product
Basic information
1 oz = 28.4 g
“Percent” means g or ml per 100 g or ml; e.g., 2% NaF solution means 2 g NaF per 100 ml water
Atomic weights: Na = 23; F = 19, Sn = 119; P = 31; O = 16
Fluoride compounds most often used are NaF, SnF2, Na2FPO3
Example 1: How much F is in 10 ml of 0.05% NaF mouthrinse?
The mouthrinse has 0.05 g of NaF per 100 ml of rinse
= 50 mg of NaF, or 5 mg of NaF per 10 ml
Amount of F = 5 × 19/42 = 2.26 mg
Example 2: How much F is in a 6.4-oz tube of Colgate MFP toothpaste? (6.4 oz = 181.8 g)
Colgate with sodium monofluorophosphate (MFP) is 0.76% Na2FPO3, so it has 0.76 g of MFP per 100 ml of toothpaste
Grams of Na2FPO3 in a 6.4-oz tube = 0.76 × 181.8/100 = 1.38 g, which is 1380 mg Na2FPO3
Amount of F in the tube = 1380 × 19/144 = 182.1 mg
BOX 26-2 Data on Toxic Fluoride Intake Levels in Humans29,69
The 10th and 90th percentiles of weight for children at various ages are as follows:
| Age | Weight |
|---|---|
| 1 year | 8-12 kg |
| 2 years | 10-15 kg |
| 3 years | 12-17 kg |
| 4 years | 14-20 kg |
| 6 years | 17-27 kg |
| 8 years | 22-34 kg |
Early work on professionally applied F began even before the first water fluoridation projects21,91 and within a few years the Knutson technique was developed.90 In this method, a 2% solution of NaF is applied in a series of four treatments over a period of several days, following an initial prophylaxis. In the 1950s the annual application of 8% stannous fluoride (SnF2) was reported to give beneficial results similar to those achieved with NaF. But staining problems were reported, and the material had an unpleasant taste. Since the early 1960s, APF has become the most widely used F compound for professional application. This material has a pH of about 3.0 and was developed after experimental work showed that the topical uptake of F by enamel was greater in an acidic environment.28 The agent has been tested in several concentrations, the most common being 1.23% F, usually as NaF, in orthophosphoric acid. The material is nonirritating and nonstaining, will tolerate the addit/>
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses