CHAPTER 26. Cervical lymphadenopathy
Dental and periodontal infections are by far the most common causes of cervical lymphadenopathy (Fig. 26.1). However, other possible causes include life-threatening diseases such as carcinomatous metastases or lymphomas. In HIV infection, lymphadenopathy is one of its most common features. Cervical lymphadenopathy without an obvious local cause is therefore a warning sign that must not be ignored.
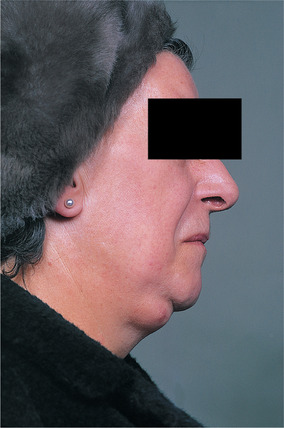 |
| Fig. 26.1
Enlarged submandibular lymph node with incipient drainage to the skin.
|
Important causes of cervical lymphadenopathy are summarised in Box 26.1, but many have been discussed in other chapters.
Box 26.1
Important causes of cervical lymphadenopathy
Infections
• Bacterial
Dental, tonsils, face or scalp infections
Tuberculosis
Syphilis
Cat-scratch disease
Lyme disease
• Viral
Herpetic stomatitis
Infectious mononucleosis
HIV infection
• Parasitic
Toxoplasmosis
• Possibly infective
Mucocutaneous lymph node syndrome (Kawasaki’s disease)
Neoplasms
• Primary
Hodgkin’s disease
Non-Hodgkin lymphoma
Leukaemia – especially lymphocytic
• Secondary
Carcinoma – oral, salivary gland or nasopharyngeal
Malignant melanoma
Other mesenchymal tumours
Miscellaneous
• Sarcoidosis
• Drug reactions
• Connective tissue diseases
Investigation
The most common cause of persistent cervical lymphadenopathy is a recent viral illness. Various clinical features provide important guides as to the likely cause of lymphadenopathy (Box 26.2).
Box 26.2
Preliminary considerations
• Patient’s age
• Localised or generalised lymphadenopathy
• Clinical characters of the nodes
• Duration of the swelling
• Associated signs or symptoms
A soft lymph node in an otherwise healthy child is unlikely to be of great significance. It is usually due to a recent viral infection and typically resolves spontaneously after a month or so. Cervical lymphadenopathy associated with generalised lymphadenopathy in a child with a sore throat and fever is likely to be due to infectious mononucleosis as discussed below. By contrast, persistent lymphadenopathy associated with anaemia in a child is likely to be due to lymphocytic leukaemia. In the older patient with a hard lymph node, a carcinoma must be suspected. Rarely, such a metastasis can form in the early stages of the disease when the primary is still small. Otherwise, a carcinoma in the anterior part of the mouth should be readily detectable by careful clinical examination. By contrast, a carcinoma of the posterior lateral border of the tongue may be difficult to visualise. Even more difficult to detect is a small primary in the nasopharynx. Various patterns of involvement of cervical lymph nodes can be produced by spread of carcinoma of the mouth (Ch. 17).
In a patient with Sjögren’s syndrome, enlargement of cervical lymph nodes may be due to infection secondary to the dry mouth, but may alternatively be due to the development of lymphoma – a recognised hazard of this disease.
Investigations for cervical lymphadenopathy where the cause is not obvious are summarised in Box 26.3. The first-line investigation should always be fine needle aspiration (FNA). This will provide an accurate diagnosis of most lymphomas, metastases and many infections including tuberculosis. Inadequate specimens or failed diagnosis should therefore trigger a re-aspiration in the first instance.
Box 26.3
Investigation of cervical lymphadenopathy
History
• Is there a history of a systemic illness?
• Has there been any contact with infectious disease (e.g. HIV or syphilis)?
• Has there been an animal scratch?
• Are there recurrent fever, lassitude, sweats or anaemia to suggest Hodgkin’s disease?
• Do any symptoms (e.g. epistaxis or hoarseness) suggest a nasopharyngeal cause?
• Are any drugs (especially phenytoin) being taken?
Examination
• Check the temperature
• Identify the node and its drainage area
• Check carefully for dental, other oral, pharyngeal or skin causes in the area
• If a possible primary cause is found (e.g. an oral ulcer) it should be biopsied
• If no local cause is found, consider ENT referral for a nasopharyngeal cause
• Examine the other side of the neck. Bilateral lymphadenopathy suggests a systemic cause
Special investigations (as appropriate)
• Blood picture (leukaemia? glandular fever?)
• Chest radiograph for mediastinal nodes (e.g. Hodgkin’s disease, sarcoidosis)
• Serology (glandular fever, toxoplasmosis, HIV)
• Angiotensin-converting enzyme and calcium levels (sarcoid)
• Mantoux test (tuberculosis)
• Fine needle aspiration (primary or metastatic neoplasm, tuberculosis)
• Thyroid scan and function tests for unsuspected thyroid tumour
• Blind biopsy of nasopharynx and tonsils if needle biopsy of the node shows malignancy
• Biopsy of node itself is a last resort. Send fresh material for mycobacterial culture
In difficult cases or when FNA fails, biopsy provides the most reliable diagnosis. Biopsy is only justified if all other investigations have proved inadequate and must be done by an expert. Biopsy may spill infectious material or malignant cells into the neck as well as produce unsightly scarring.
TUBERCULOUS CERVICAL LYMPHADENOPATHY → Summary p. 172
Tuberculous infection of the cervical lymph nodes is a primary infection which accounts for less than 10% of tuberculosis in Britain. Mycobacterium tuberculosis (human) accounts for over 90% of isolates. Patients are mostly adults, mainly immigrants of Asian or Afro-Caribbean origin. Nontuberculous (‘atypical’) mycobacteria, particularly M. avium intracellulare or M. scrofulaceum, are now the major cause of cervical mycobacterial lymphadenitis in immunocompetent children. As discussed earlier, the incidence of mycobacterial infections is growing and multiply-resistant strains are becoming widespread.
Clinical features (Box 26.4)
Box 26.4
Typical clinical features of tuberculous lymph nodes
• Firm swelling, usually of a group of nodes
• Nodes typically become matted
• Abscess or sinus formation, if neglected
• Calcified nodes from past, healed disease (Fig. 26.2)
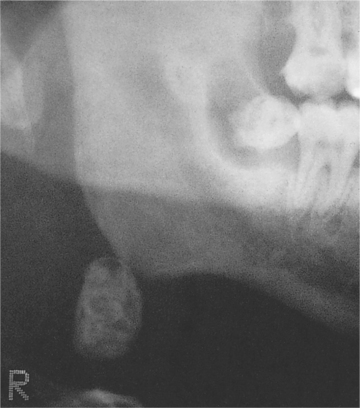 |
| Fig. 26.2
Tuberculosis. Calcified cervical lymph node seen in a panoramic tomogram. Calcified nodes are often multiple and indicate past rather than active infection.
(By kind permission of Mr EJ Whaites.)
|
Pathology
Diagnosis depends on finding granulomas (Fig. 26.3) in which mycobacteria should be demonstrable by Ziehl–Neelsen staining or by immunofluorescence using auramine-rhodamine. However, infection by nontuberculous mycobacteria, even in immunocompetent children, is likely to give rise to poorly-defined or irregular granulomas without palisading, or to ill-defined aggregates of epithelioid histiocytes. Caseation may be absent (Fig. 26.4), but there may be areas of necrosis with numerous neutrophils. Ziehl–Neelson staining is also negative.
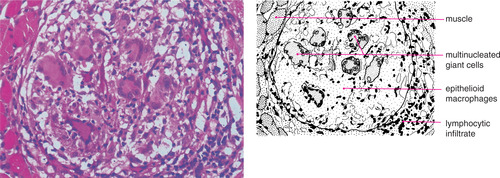 |
| Fig. 26.3
Tuberculosis. Numerous multinucleate Langhans giant cells are conspicuous in this granuloma.
|



