25 Root Canal Disinfection
 Disinfection
Disinfection
 Ultrasonically Activated Irrigation
Ultrasonically Activated Irrigation
 New Irrigation and Disinfection Systems
New Irrigation and Disinfection Systems
 Possible Adverse Effects of Root Canal Irrigation
Possible Adverse Effects of Root Canal Irrigation
 Mechanical Irrigation with RinsEndo
Mechanical Irrigation with RinsEndo
 Endox
Endox
 Electrochemically Activated Water (ECA)
Electrochemically Activated Water (ECA)
 Photo-activated Disinfection (PAD)
Photo-activated Disinfection (PAD)
 Ozone
Ozone
 Noninstrumentation Technique (NIT)
Noninstrumentation Technique (NIT)
Disinfection
Disinfection of the root canal system using solutions and substances with antibacterial and tissue-dissolving properties—the so-called “chemical debridement”—is a critical component of root canal preparation and instrumentation. This procedure is therefore often also referred to as “chemo-mechanical” preparation. Adequate disinfection is critical for successful completion of any endodontic therapy.
In cases of pulpal necrosis, in which there is bacterially induced tissue destruction, 106–108 microorganisms per milliliter of canal content have been observed; these are primarily anaerobic organisms. In cases of endodontic failure, mainly Gram-positive, facultative anaerobic bacteria, in particular Enterococcus faecalis, and various fungi have been identified (Siqueira and Rôças, 2006).
A proportion of the bacteria occupy the endodontium in the planktonic form; the other portion is incorporated into a biofilm attached to the root canal wall. (A biofilm is a single- or multicellular layer of microbes embedded in an extracellular matrix.) The microorganisms within the biofilm can be quite resistant to the host immune response as well as to antibiotic therapy. Individual microorganisms can be released from the biofilm and maintain the level of infection (Svensäter and Bergenholtz, 2004).
In cases of pulpitis with irreversibly inflamed but still vital and vascularized tissue, which is in transition to necrosis, microorganisms are seldom observed within the root canal system. Irrigation of the canal during instrumentation dissolves and removes both tissue remnants and dentinal debris.
The primary goal of chemo-mechanical preparation of the root canal system is the elimination of intracanal microflora as well as necrotic and/or infected canal contents, and in the case of residual vital pulp the removal of all soft tissues. It has been demonstrated time and again that mechanical root canal instrumentation alone does not eliminate all microflora (Peters, 2004; Haapasalo et al., 2005; Hülsmann et al., 2005). In other words, purely mechanical instrumentation of the root canal cannot sufficiently reduce the bacterial “load” to permit healing and/or prevent apical periodontitis. Additional chemical disinfection measures are required, which are included under the umbrella term “root canal irrigation.”
Any discussion of, or debate about, particular disinfection strategies is directly dependent on contemporary microbiologic knowledge. Improved methods for the identification of specific microorganisms have recently enhanced our knowledge of the microbiology of an infected root canal system. A direct consequence of this new knowledge is the onus on the profession to seek new strategies for combating the complex microflora. For this reason, it is to be expected that the currently recommended disinfection protocol will (have to) be amended in the near future.
Possibilities and Limitations of Root Canal Disinfection
Numerous in vitro studies have demonstrated that a stringent antiseptic treatment protocol will lead to significant reduction of microbiologic contamination of the root canal. Through mechanical instrumentation and saline irrigation alone, the number of microorganisms within the canal can be reduced from approximately 106–108 microorganisms per milliliter of canal content by a factor of 100–1000 (Bystroem and Sundqvist, 1983). In addition to the removal of necrotic tissue, root canal preparation must also include the introduction of a sufficient volume of irrigating solution into the apical portions of the root canal system. The use of even the thinnest irrigation needle requires preparation to approximately ISO size 35. When sodium hypochlorite is used as an irrigant, approximately 33% of all root canals have been shown to exhibit no residual microorganisms. Increasing the concentration to 5% did not significantly reduce the bacterial count. With the addition of calcium hydroxide the number of bacteria-free root canals increased to 97% (Bystroem et al., 1985). However, consistently reproducible and predictable elimination of microbial contamination has not yet been achieved with any disinfection technique or solution. If a root canal is instrumented and thoroughly irrigated, and then left unfilled but covered with a coronal filling that aims to provide a bacteria-tight seal, within a week the microorganisms will recolonize to re-establish the initial levels within the root canal.
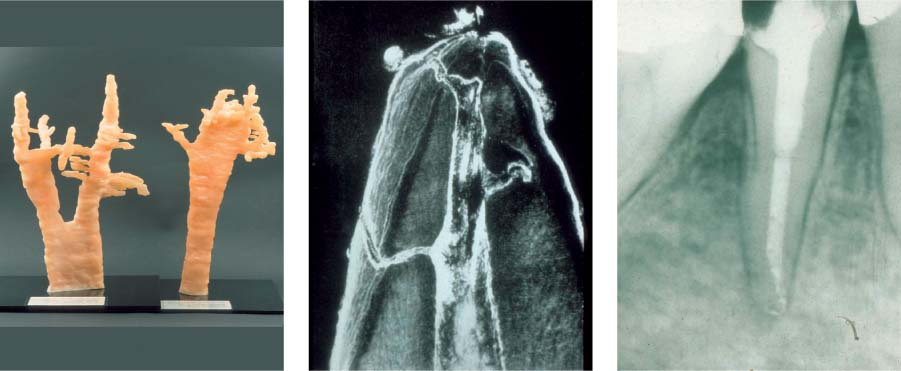
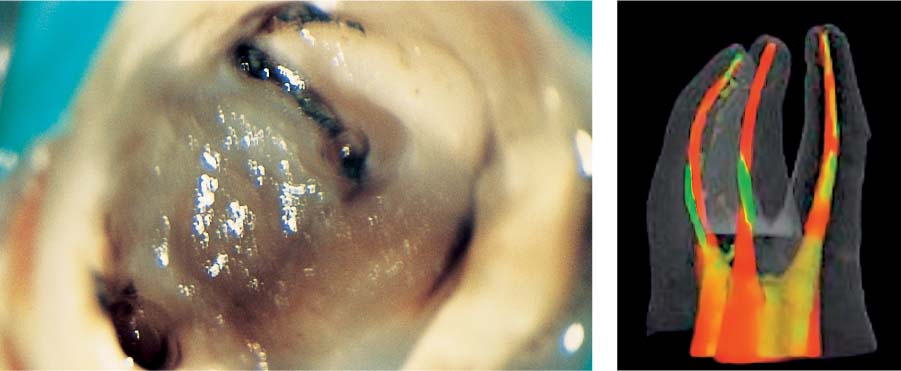
25.1 Complexity of the endodontic system
Left: Three-dimensional reconstructions of the root canal systems of a maxillary canine and premolar.
Middle: This histologic specimen clearly demonstrates that some areas of the complex root canal system can only be cleaned chemically and not mechanically.
Right: Following dissolution of soft tissues by means of irrigants, even the areas that could not be instrumented (e.g., lateral canals) were partly successfully obturated.
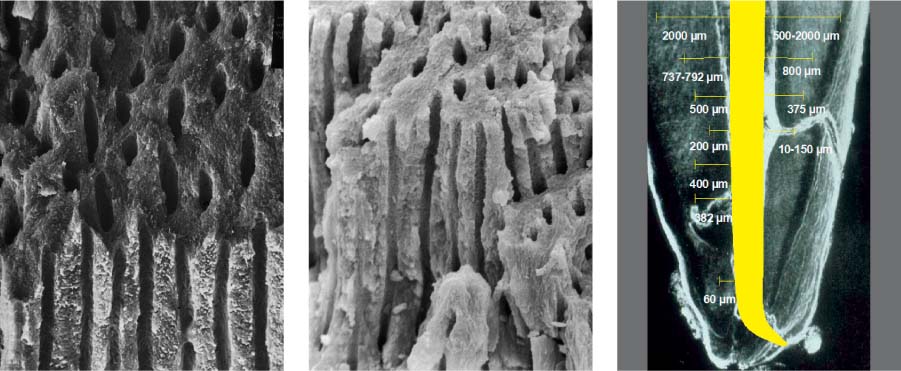
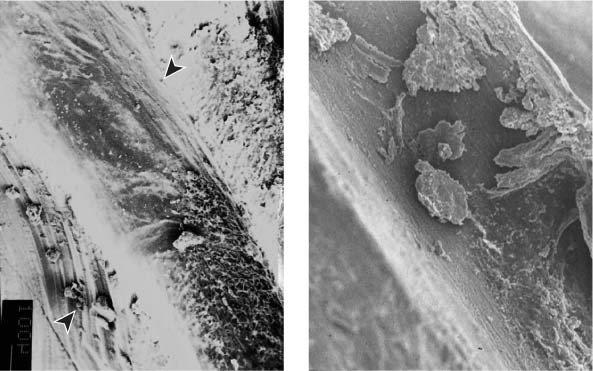
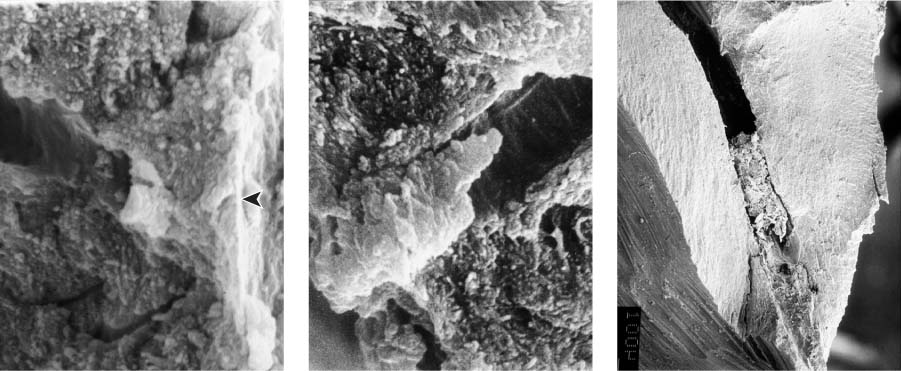
25.2 Dentin structure and infection
Left: From this dentin specimen it is evident that the dentinal tubules are an integral component of the endodontic system, and are clearly susceptible to bacterial colonization.
Middle: This dentin specimen clearly shows the large numbers of open lacunae within dentin, all of which are vulnerable to microbial colonization (magnification ×2000).
Right: Depth of dentin infection as shown in numerous research studies.
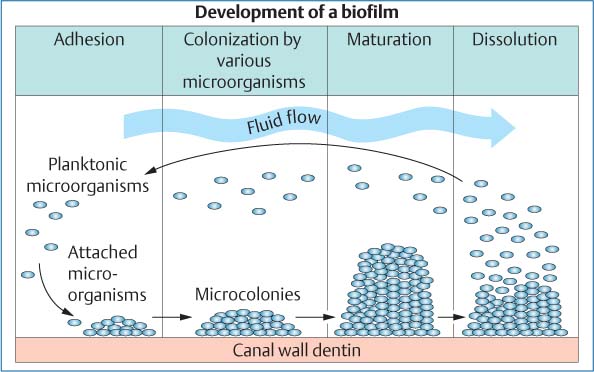
25.3 Biofilm
The growth of a bacterial biofilm can be divided into various phases: Following attachment of planktonic bacteria onto the canal wall dentin and the formation of an extracellular matrix, a multilayered, 3D biofilm develops. With detachment of microorganisms from the superficial layers, the cycle of biofilm growth is repeated.
Adequate disinfection of the root canal leading to successful endodontic treatment can only be achieved by a carefully determined series of individual clinical procedures: Application of rubber dam – disinfection of the clinical working field – sterile instruments – adequate size of the access cavities – adequate irrigating solutions, irrigating technique, irrigating time, irrigating depth – antimicrobial intracanal dressing – impermeable root canal obturation – impermeable temporary and definitive restorations, as well as prevention of coronal leakage.
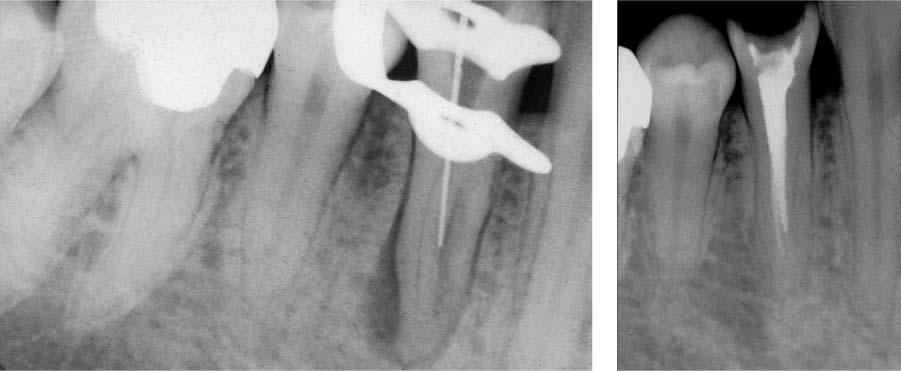
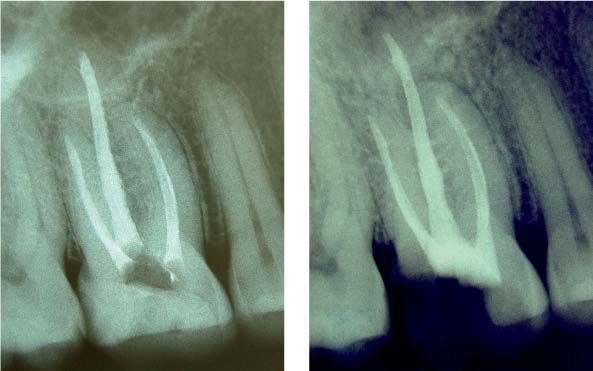
25.4 Clinical effects
The two root canals were not able to be completely instrumented mechanically.
Right: With intensive chemical disinfection, the microbiologic load was sufficiently reduced, leading to complete healing of the periradicular lesion.
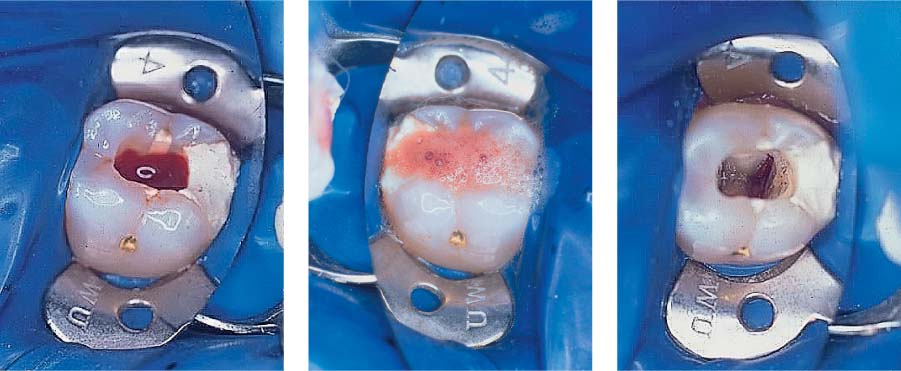
25.5 Tissue remnants
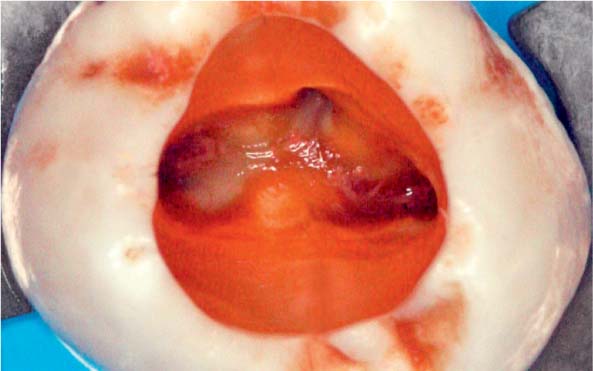
Left: After accessing the tooth with a necrotic pulp, magnification ×25 under the clinical microscope revealed tissue remnants within the root canal orifices.
Right: The superimposition of preand postoperative structures of the endodontic system clearly reveal that areas of the root canal wall have either not been instrumented or have been only partially instrumented (courtesy of Dr. F. Paqué).
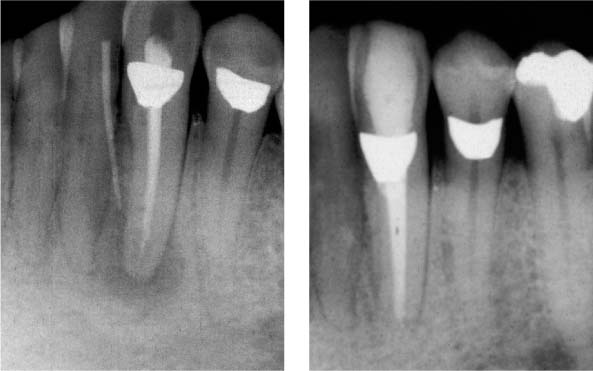
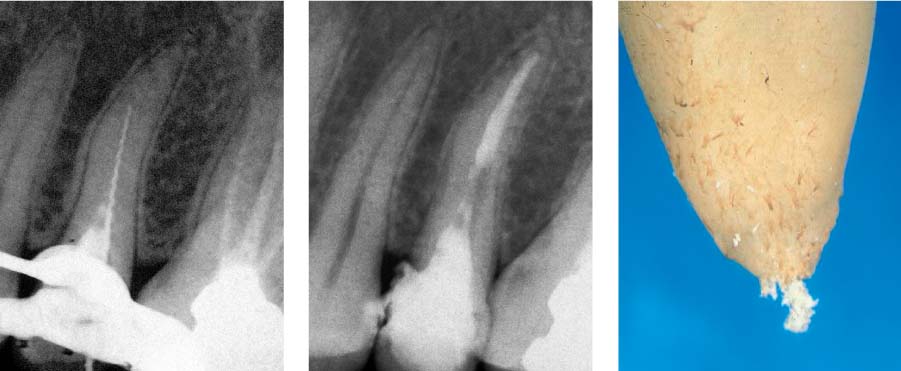
25.6 Failure
Left: The radiograph reveals a homogeneous root canal filling. The periapical tissues radiographically appear free of inflammation.
Right: Loss of the definitive restoration led to bacterial recolonization of the root canal and development of apical periodontitis (5 months following root canal obturation).
Disinfection Protocol
Optimum disinfection of the endodontic system will be determined by the initial clinical findings as well as by the suspected extent of microbial contamination. The most important clinical point is to differentiate between infected and noninfected root canals.
A disinfection protocol for a noninfected endodontic system must consider the following clinical observations:
• There are no microorganisms in the deeper levels of the inflamed pulpal tissue. The primary purpose of initial chemical disinfection is to eliminate and remove any soft tissue that was not mechanically removed.
• Irrigation of the root canal assists in removal of dentinal debris created by the instrumentation.
• Irrigation of the root canal should remove any smear layer created by the mechanical instrumentation of the root canal walls.
25.7 Noninstrumented root canal wall region
Horizontal sections before and after instrumentation: a smooth and complete preparation of the lateral extensions of the root canal was not achieved.
25.8 Debris
Left: Virtually every canal has non-instrumented sections (arrow), even after intensive root canal preparation.
Right: Following instrumentation there is clear evidence of remaining debris, underneath which a smear layer can be observed (magnification ×200).
25.9 Debris
Massive accumulation of debris following mechanical instrumentation with insufficient removal of dentin and soft tissues.
• A deep bacterial infection is present in the endodontium, including the dentinal tubules. The precise depth of infection cannot be determined clinically. Mechanical instrumentation of the root canal will only remove a portion of the infected tissues and the infected dentin on the root canal wall. The bacterial “load” can thus be reduced by a factor of 1000–10 000 (Bystroem and Sundqvist, 1981).
• The irrigant must be capable of neutralizing bacterial metabolic products (lipopolysaccharides) within the dentin.
• A smear layer will remain following mechanical instrumentation of the root canal walls. The selected irrigant must be effective against the organic as well as the inorganic components of the smear layer.
The disinfection protocol for retreatment must consider that the bacterial spectrum is most probably different from that of primary endodontic disease and in some cases will consist of micro-organisms that are resistant to “traditional” disinfection strategies (e.g., Candida species, Actinomyces, E. faecalis).
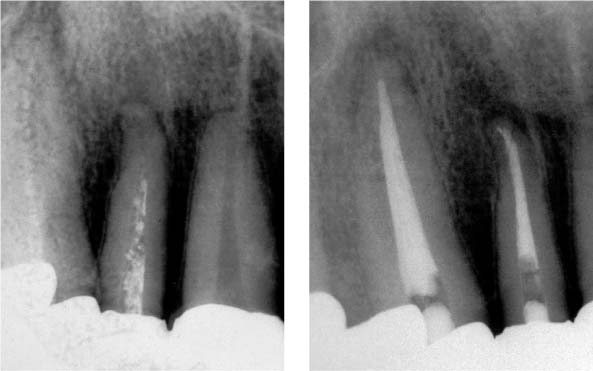
25.10 Retreatment
Healing of the periradicular lesion 1 year following disinfection of the endodontic system.
25.11 Retreatment
Removal of the root canal filling material, disinfection, and complete reobturation of the root canal system.
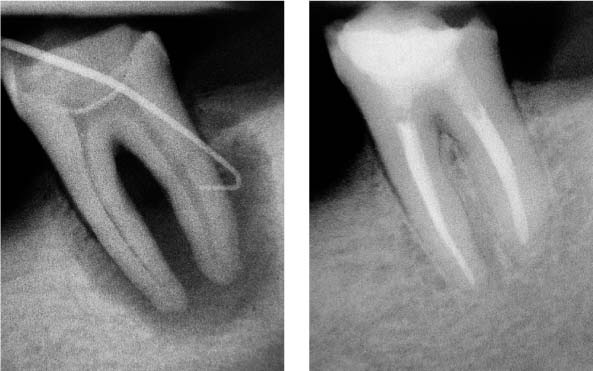
25.12 Apical periodontitis
Complete healing of the lesion within 1 year demonstrating sufficient disinfection of the root canals.
Debris and Smear Layer
Debris is defined as loose accumulations of dentinal chips and remnants of vital or necrotic soft tissues on the root canal wall. Mechanical instrumentation results in the formation of a smear layer on the root canal wall, which can penetrate deeply into the dentinal tubules. The smear layer consists of tissue remnants, cellular debris, dentinal chips, bacteria and bacterial remnants (Koçkapan, 1995; Sen et al., 1995).
The smear layer not only reduces the permeability of the dentin but also prevents secure adaptation of the root canal filling to the root dentin.
If irrigation of the root canal during the process of mechanical instrumentation is inadequate, a complete blockage of the root canal system can occur, with associated loss of actual working length because of the apical accumulation of dentinal chips and tissue remnants; also, debris from previous coronal restorations can be inadvertently transported towards the apex.
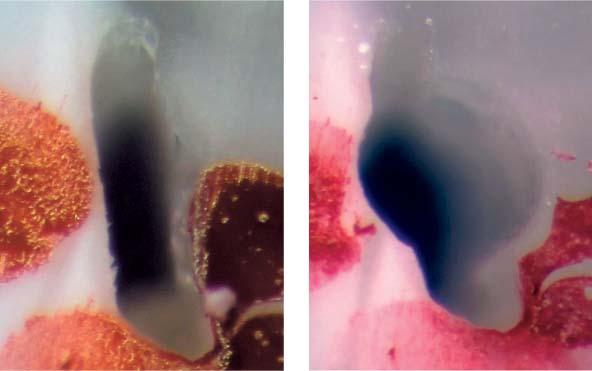
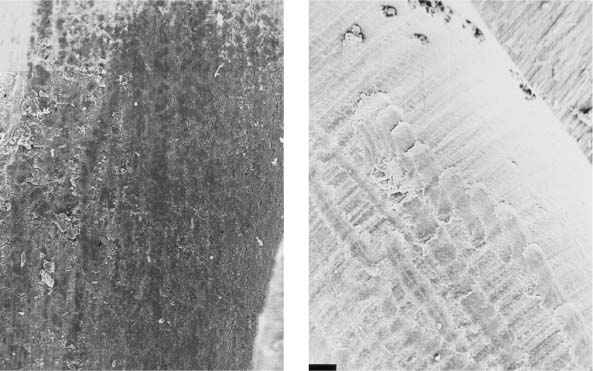
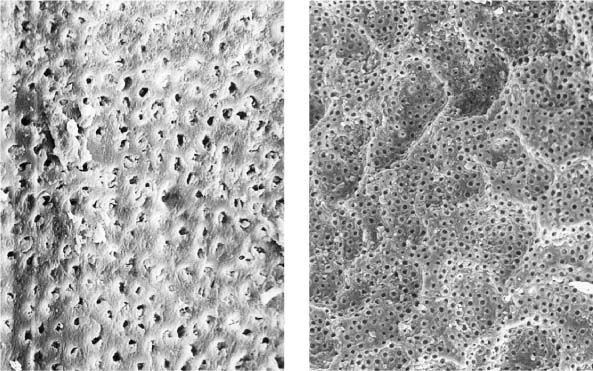
25.13 Smear layer
Left: Following mechanical instrumentation, the root canal wall is covered by a smear layer; no open dentinal tubules are seen (magnification ×1000).
Right: The root canal wall is covered by a smear layer on which additional debris is loosely distributed (magnification ×200).
25.14 Smear layer
Left: The smear layer (arrow) appears attached to the root canal wall, and has been forced into the dentinal tubules (magnification ×3000).
Middle: A dentinal tubule clearly blocked by components of the smear layer (magnification ×10 000).
Right: If dentinal debris is not regularly removed by irrigation, the endodontic instrumentation will tend to force such debris apically, leading to irreversible blockage of the root canal (magnification ×100).
25.15 Apical blockade
Left: Maxillary premolar with a ledge on the outer curvature of the root canal, as well as apical blockade of the root canal. The ledge is probably the result of an attempt to eliminate the blockade by using rigid instruments.
Middle: By means of careful instrumentation and intensive irrigation, it was possible to re-establish the patency of the root canal.
Right: Debris forced through the apical foramen, which can lead to postoperative pain.
The goals of irrigation of the root canal system are as follows:
• Antibacterial action with disinfection of the endodontic system.
• Dissolution and removal of all organic and inorganic canal contents, even from those areas of the complex endodontic system that are not accessible to mechanical instrumentation. This procedure leads to the removal of any substrate on which the remaining bacterial might survive.
• Inactivation of bacterial lipopolysaccharides and dissolution of the biofilm.
• Removal of dentinal chips and debris, therefore preventing any blockade of the root canal, and removal of the smear layer.
• Highest possible tissue compatibility, lowest possible cytotoxicity
• There should be no neutralization of the substances used, no alteration of the characteristics of the hard tissues of the tooth, and no adverse impact on postendodontic restoration (Ørstavik, 2003; Hapasaalo et al., 2005; Hülsmann, 2006).
25.16 Sodium hypochlorite (NaOCl)
Left: Profuse pulpal hemorrhage after opening of the coronal pulp chamber in a case of irreversible pulpitis.
Middle: Using a NaOCl-soaked cotton pellet the bleeding has been stopped.
Right: Following cessation of bleeding, a thorough inspection of the pulp cavity is possible.
25.17 Irrigation with sodium hypochlorite
Left: Well-cleaned dentin that is free of soft-tissue remnants (magnification ×250).
Right: Clearly visible root canal wall completely cleared of soft-tissue remnants in a root canal that was not mechanically instrumented (magnification ×500).
25.18 Chlorhexidine digluconate (CHX)
The combination of NaOCl and CHX leads to a brownish discoloration due to formation of parachloraniline.
Irrigants
The most commonly used irrigants are:
• Sodium hypochlorite: This has an effective antimicrobial action against a large variety of endodontically relevant microorganisms. It does not, however, in every case achieve a predictable and/or reproducible elimination of microbiologic infection. With regard to tissue compatibility, concentrations of 1–3% are recommended (Dammaschke, 2003; Ørstavik, 2003).
• Hydrogen peroxide: This dissolves only vital tissue and has less of an antibacterial effect than sodium hypochlorite. No synergistic effect has been demonstrated, so this solution has been eliminated from endodontic irrigation protocols.
• EDTA: Chelators are capable of removing the smear layer and therefore opening the dentinal tubules. Irrigation with EDTA (15–17%) is recommended following instrumentation to enhance the effect of sodium hypochlorite and calcium hydroxide (Heckendorff and Hülsmann, 2002).
• Chlorhexidine: The 2% concentration is recommended for root canal irrigation when problematic microorganisms such as E. faecalis are suspected to be present within the root canal (e.g., retreatment procedures). Chlorhexidine does not have any tissue-dissolving properties, and can therefore not replace sodium hypochlorite (Safavi and Spångberg, 2006).
25.19 Chelators
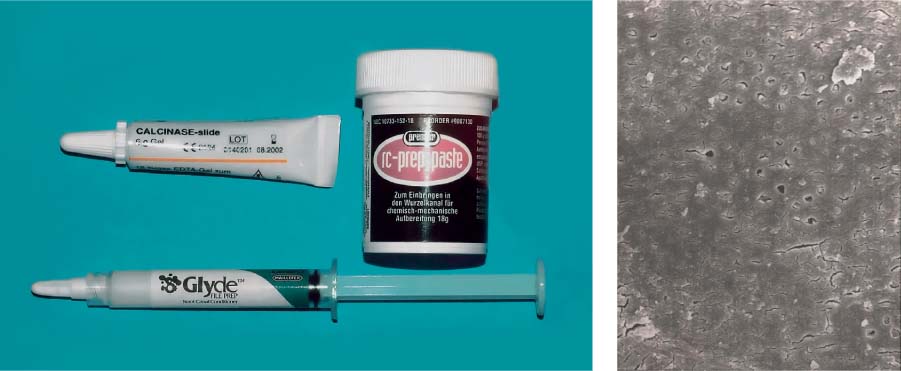
Left: EDTA preparations in the form of a paste are recommended for use during instrumentation.
Right: Dentin surface following application of a chelator paste. The smear layer has been removed and the openings of the dentinal tubules reveal an early stage of demineralization (magnification ×1000).
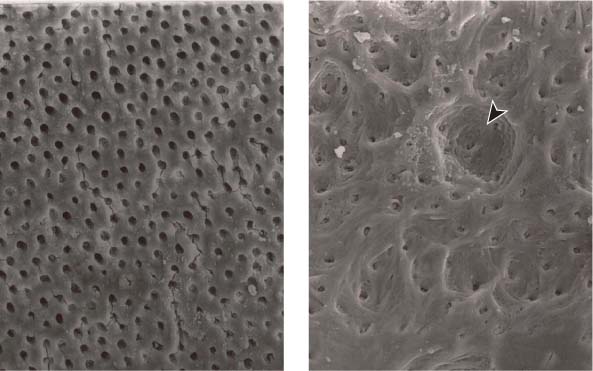
25.20 Dentinal erosion with use of EDTA
Left: Surface of the root canal wall following irrigation with EDTA. The smear layer has been completely removed and the dentinal tubules are clearly open (magnification ×1000).
Right: With extended exposure to EDTA, erosion of the intracanal dentin can be observed (arrow) (magnification ×1000).
25.21 MTAD
MTAD irrigating solution consists of citric acid, doxycycline, and a detergent, and is recommended for the final irrigation following mechanical instrumentation.
Irrigation Technique
The temperature, the amount of the irrigant used, and the irrigation technique are important factors that influence the efficacy of disinfection. Use of large volumes of the irrigation solution (10–20 mL per root canal) and continuous replacement with fresh solution leads to an improved reduction of bacteria. Heated sodium hypochlorite (45–60°C) dissolves the tissue slightly better and has an improved antibacterial effect, but heating also causes a more rapid decline of the solution’s effectiveness.
In addition, it is important to consider the depth of penetration of the irrigation needle (working length minus 2–3 mm, without binding) and the effective contact time of the irrigant with the biofilm (minimum 30 minutes is required) (Kahn et al., 1995; Hülsmann 2006).
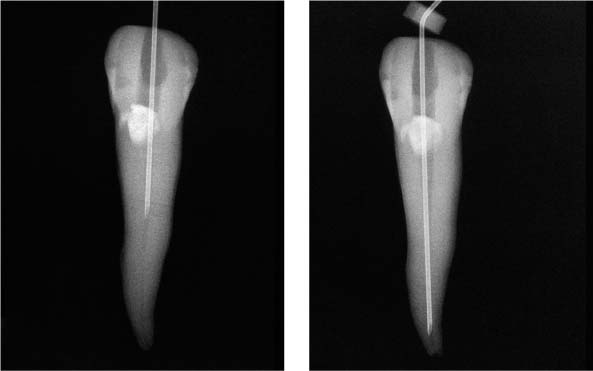
25.22 Irrigation technique
Extremely small-diameter irrigation needles (middle: NaviTip, right: US-needle) can be inserted much deeper than conventional injection needles (left). It is clear from these radiographs that the acute tip prohibits deeper penetration of the injection needle.
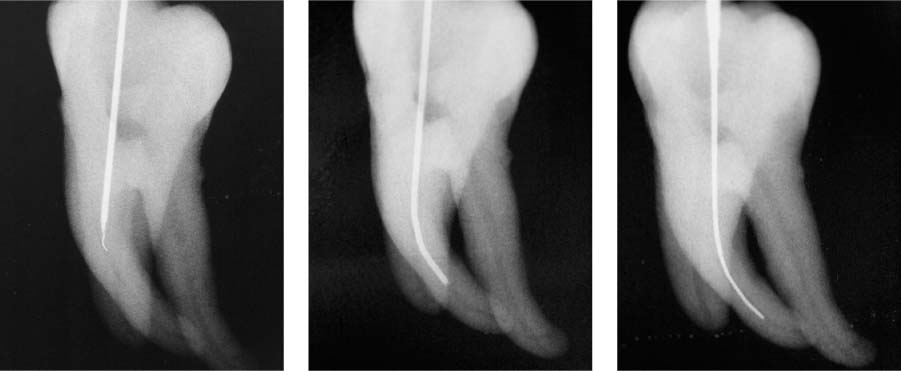
25.23 Irrigation technique
As the root canal preparation procedure enlarges the canal, the depth of penetration of the irrigation needle increases with increasing instrument sizes.
25.24 Irrigation technique
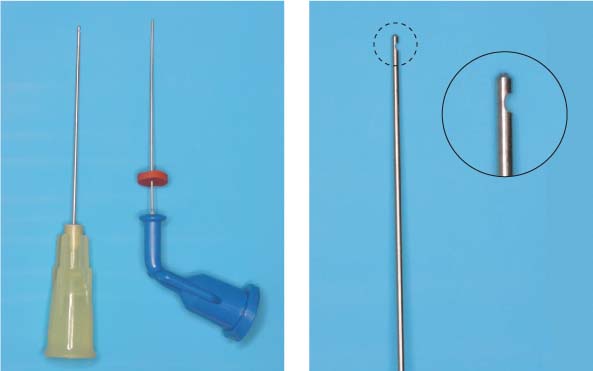
Small-diameter irrigation needles.
Left: Maxi-Probe tip (Hawe-Neos, Bioggio, Switzerland) and NaviTip (Ultradent, South Jordan, UT, USA), marked with a rubber stopper to control the insertion depth. The external diameter corresponds to ISO size 30 and ensures deep penetration of the irrigating solution.
Right: The Maxi-Probe needle has a blun/>
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses