Chapter 23
Adhesive Restorative Materials: Bonding of Resin-based Materials
23.1 Introduction
The development and regular use of adhesive materials has begun to revolutionize many aspects of restorative and preventive dentistry. Attitudes towards cavity preparation are altering since, with adhesive materials, it is no longer necessary to produce large undercuts in order to retain the filling. These techniques are, therefore, responsible for the conservation of large quantities of sound tooth substance which would otherwise be victim to the dental bur. Microleakage, a major dental problem which is probably responsible for many cases of secondary caries, may be reduced or eliminated. New forms of treatment, such as the sealing of pits and fissures on posterior teeth, the coverage of badly stained or deformed teeth in order to improve appearance and the direct bonding of brackets in orthodontics have all grown from the development of adhesive systems.
Section 2.5 deals briefly with the general mechanistic aspects of adhesion. Three major approaches can be identified. (1), bonding through micromechanical attachment; in dentistry this is best illustrated through the bonding of resins to enamel using the acid-etch technique. (2), bonding through chemical adhesion to either enamel or dentine can be identified in many systems based on the use of coupling agents or cements containing polyacids. (3), bonding through a complex mechanism involving wetting, penetration and the formation of a layer of bound material at the interface between the restorative and the substrate. The latter describes the mode of action of many modern dentine bonding agents.
23.2 Acid-etch systems for bonding to enamel
The surface of enamel is smooth and has little potential for bonding by micromechanical attachment. On treatment with certain acids, however, the structure of the enamel surface may be modified considerably. Figure 23.1 shows the surface of human enamel following one minute of etching with a 37% solution of phosphoric acid, which is the acid of choice for most applications of the acid-etch technique. Solutions of phosphoric acid are difficult to control when applied to enamel, some acid inevitably contacts areas which are not required to be etched. One improvement in acidetching procedures has been the development of acidified gels. These contain phosphoric acid in aqueous gel which is viscous enough to allow controlled placement in the required area. In addition, the gel is normally pigmented, a feature which further aids control.
The pattern of etching enamel can vary. The most common (type 1) involves preferential removal of the enamel prism cores, the prism peripheries remaining intact. The type 2 etching pattern is the opposite of the type 1, involving preferential removal of the peripheries with the cores being left intact. The type 3 etching pattern contains areas which resemble both type 1 and type 2 along with some less distinct areas where the pattern of etching appears to be unrelated to the enamel prism morphology.
Fig. 23.1 Scanning electron micrograph of the surface of enamel after etching with 37% phosphoric acid followed by rinsing and drying (×2000 magnification).

The individual features evident in Fig. 23.1 correspond to the ends of enamel prisms, each being about 5 μm in diameter. This surface is now suitable for micromechanical attachment since it contains a myriad of small undercuts into which resins can gain ingress, set and form a mechanical lock. The three major factors which affect the success or failure of acid-etch bonding systems are as follows:
The type of resin applied to the etched enamel surface depends upon the specific application being used. For composite resins the mixed material may be applied directly to the etched enamel surface. Resin from the composite flows into the etched enamel and sets, forming rigid tags, typically 25 μm long, which retain the filling. Many manufacturers supply a fluid bonding resin which may enhance the adhesive bond strength (see Fig. 23.2). It consists of a resin similar to that used in the composite material but contains no filler particles. It is very fluid and readily flows into the etched enamel surface. The bonding resin may be a single component which is activated by light or may consist of two fluid resins, one containing initiator and the other activator, which require mixing before being applied to the etched enamel. The composite filling material is applied directly to the surface of the bonding resin. The need for the use of the intermediary layer of unfilled bonding resin varies depending upon the type of composite material used. For conventional composites it is likely that the materials contain sufficient excess resin to satisfy the requirements for attachment to etched enamel without the presence of the intermediate resin. For the more heavily filled and viscous products (mainly hybrid-type composites) it is necessary to use the unfilled resin layer in order to achieve adequate penetration of the etched enamel surface.
Fig. 23.2 Pack of enamel bonding agent containing etching agent (37% phosphoric acid) and two fluid resins which begin to polymerize on mixing. Modern materials contain a single resin which is light activated.
Bonding readily occurs at the unfilled resin to composite interface. This is aided by the fact that surface layers of resins polymerised by a free radical mechanism remain soft and unpolymerised due to the inhibiting effect which oxygen has on the polymerisation mechanism. On applying a composite material to the surface of a ‘cured’ unfilled resin, mixing of the two resin systems occurs at the interface followed by a degree of copolymerisation and entangling which effectively bonds the filled and unfilled resins together. The resulting shear bond strength achieved between etched enamel and restorative resins is 16–20 MPa.
The way in which the enamel etch pattern affects bonding has never been conclusively proven. The nature of some enamel bonding systems has changed over recent years as some manufacturers have produced materials which can be used for both enamel and dentine bonding. Hence, some enamel bonding resins now contain primers and solvents which enable bonding to moist enamel to be achieved. This is contrary to the previous situation in which thorough drying of enamel was essential for effective bonding. This theme is developed further in Section 23.8.
23.3 Applications of the acid-etch technique
The acid-etch technique has many applications in dentistry. It is now widely used for most composite fillings as a means of aiding retention and reducing or preventing microleakage. For class IV cavities (incisal edge restorations) the acid-etch technique has replaced the gold inlay as the treatment of choice for restoring the tooth contours and function. In this example the use of an adhesive system allows the conservation of considerable quantities of tooth substance which would otherwise be lost in cavity preparation. Bonding of resins using the acid-etch technique has also been used as a means of strengthening or splinting teeth which have been weakened by cavity preparation. It can readily be shown that a tooth having a prepared cavity is weakened relative to an unprepared tooth. Under stress, fracture of the tooth is likely with cusp fracture being the most likely occurrence. Restoring the cavity with a non-adhesive restoration has little beneficial effect on the strength of the tooth whereas the use of an adhesive material will strengthen the tooth and help to prevent cusp fractures.
Fissure sealants are now widely used for preventing pit and fissure caries. The majority of products are based on dimethacrylate resin systems such as Bis GMA or urethane dimethacrylate. The simplest products consist of two liquid components, each containing the dimethacrylate monomer or a mixture of the monomer and a diluent monomer such as triethylene glycol dimethacrylate (see Fig. 22.4). In addition, one component contains a peroxide initiator whilst the other contains an amine activator. The normal procedure is to mix together one drop of each liquid component in order to activate the polymerisation of the methacrylate groups. Chemically, these products are almost identical to the intermediary resin bonding agents referred to in the previous section. The mixed material is applied to the etched enamel of the occlusal surface of the selected tooth where it typically takes a few minutes to harden. The surface layer remains tacky due to air inhibition of the polymerisation and is generally wiped away to expose the fully cured material underneath.
Some products contain additives such as titanium dioxide in order to make the sealant more readily visible in situ (Fig. 23.3). With the unpigmented material the sealant can be difficult to detect on inspection due to the translucent nature of the resin. There has also been a trend towards adding glass filler to improve durability and those products containing filler may be considered as lightly filled composites. The filler content remains somewhat lower than that found in composite filling materials so that the viscosity is low enough to enable the materials to flow into the fissure pattern of the occlusal surface of the tooth.
Not surprisingly, the development of light-activated fissure sealant materials has followed the development of light-activated composites. One of the most popular materials in use until a few years ago was activated using ultraviolet radiation. This is no longer used and has been replaced by products which are activated by light within the visible range. The visible light activation units which are used for curing composites can also be used to activate curing of fissure sealants so it is convenient for dentists who have such a unit to use it for several applications (see p. 204). The problems of limited depth of cure do not apply to these materials which are used in thin sections. The efficacy of fissure sealants is measured in one of two ways. One way is to monitor the survival of the sealant as a function of time. Apparent sealant loss may be due to detachment or wear. The other way is to monitor the caries reduction on sealed teeth compared with a group of unsealed control teeth. Surprisingly the two approaches do not produce the same result. Sealants, being unfilled or lightly filled resins, are relatively soft and readily undergo abrasive wear, although this is minimized in practice due to the fact that the material is in a protected environment in which it is unlikely to come under direct occlusal loads. In any event, wear of the sealant does not impair its efficacy since the surface enamel remains impregnated with resin. Likewise, if a sealant becomes detached it may still have some beneficial effect if it leaves behind an enamel surface which is resin impregnated.
Fig. 23.3 Pack of fissure sealant material which is similar in composition to the enamel bonding agent shown in Fig. 23.2. In this product one resin contains titanium dioxide in order to allow the sealant to be visualized in the pits and fissures after placement. Equivalent materials, provided as a single liquid resin component, are light activated.

The success of fissure sealants is mainly dependent on initial placement conditions and techniques. In order to get good resin tag formation the enamel must be properly etched and washed and thoroughly dried before the sealant is applied. It is the variability in levels of moisture which causes the wide variation in success rates recorded for fissure sealants. Attempts have been made to produce fluoride containing sealants so that the benefits of surface sealing can be combined with those of a sustained fluoride release. Release of fluoride from resin-based materials is difficult to achieve and the rate of release is generally much lower than is observed for glass ionomers. Fluoride releasing resin-modified glass ionomers (see Chapter 25) offer a potential for achieving the ideal of effective bonding durability and sustained fluoride release.
Most recently, chemically active luting resins have become available which will bond to oxide layers on the surface of non-precious metals and to copper oxide on heat treated gold casting alloys (see Section 23.9).
Resin systems are now widely used for attaching orthodontic brackets. These resins are normally supplied as two components carrying relatively high loadings of initiator and activator respectively. One component is applied to the etched enamel surface and the other to the bracket. When the two are pressed together rapid setting takes place. Alternatively, conventional composite resin materials can be used for this application.
Composites are gaining in popularity for the attachment of bridges. This involves a more conservative technique than the traditional methods, which involve considerable destruction of the abutment teeth in order to achieve retention. The principle of the resin-bonded systems is that the composite bonds mechanically to the etched enamel of the tooth and also to the surface of the cast alloy framework of the bridge. There are various means of achieving mechanical attachment between the resin and the alloy. One system employs the use of perforated ‘wings’ on the cast alloy bridge framework. The composite used for bonding flows through the perforations giving a mechanical lock onto the framework. Attachment to the abutment tooth is through resin penetration of acid-etched enamel. This type of bridge is known as a Rochette bridge. Another approach is to etch the wings of the bridge to produce a roughened surface with a myriad of small undercuts similar in appearance to the surface of etched enamel (Fig. 23.4). This surface is suitable for achieving mechanical attachment with a composite. Etching is carried out electrolytically or in a strong acid. This second type of bridge is normally referred to as a Maryland bridge. Chemically active resins capable of attaching to both metals and tooth have been used to bond sand-blasted metal retainers to the supporting tooth. (see Fig. 23.5)
Fig. 23.4 A scanning electron micrograph of an electrolytically acid-etched metal surface showing the irregular etch pattern that can give micromechanical retention for attachment (×124).
Fig. 23.5 A chemically active resin luting cement which is used to bond laboratory made dental appliances and restorations to teeth.
The active groups may be phosphate or melittic acid anhydride groups akin to those used in certain dentine bonding agents (see Section 23.4).
Another application of the acid-etch technique is the attachment of acrylic or porcelain labial veneers in order to improve the appearance of stained, discoloured or misshapen teeth. Acrylic veneers may be produced in a range of standard shapes and sizes which can be customized by heat adaptation to a cast of the patient’s teeth. A composite of the correct shade is used to attach the veneer to the etched enamel surface. The fitting surface of the veneer is softened with a solvent primer to aid bonding between the veneer and composite. The acrylic laminate veneers have limited lifetime due to the fact that the acrylic resin is soft and readily abrades to expose regions of the underlying composite resin. Also the bond between the composite resin and veneer has a tendency to fail, probably due to stresses set up at the interface as a result of the differing values of the coefficient of thermal expansion and water sorption for the two materials. The use of porcelain veneers is covered in Section 11.8. Essentially the veneers are custom made and bonding to the composite is achieved by etching the fitting surface of the veneer with hydrofluoric acid and using a silane coupling agent to chemically link silica groups in the porcelain to methacrylate groups in the resin. Porcelain veneers are much harder and more resistant to abrasion than acrylic veneers. Providing that etching and silane treatment is carried out carefully the bond to composite appears adequate. The brittle nature of the porcelain must be taken into account when considering the design of veneers if chipping at the incisal edge is to be avoided.
23.4 Bonding to dentine – background
The mechanism of bonding to enamel involves the penetration of resin into the relatively porous surface layer of the etched enamel to create a mechanical interlocking. It was recognized many years ago that a similar mechanism could potentially be used with dentine. This would involve etching of the surface of exposed dentine with acid to expose the patent dentinal tubules which could be penetrated by resin to form tags. Until quite recently this mechanism of bonding to dentine was rejected by most authorities as being both ineffective and unacceptable for the following reasons:
Attempts at chemical bonding
When attempting to form chemical links with the tooth surface it is obviously necessary to pay some attention to the chemical nature of the substrate tooth material. Dentine contains approximately 50% hydroxyapatite and 30% polypeptides (e.g. collagen), the balance being aqueous solutions which occupy the dentinal tubules. Enamel, on the other hand, contains only about 1% protein and 97% hydroxyapatite. Forming chemical links with enamel therefore inevitably involves forming a union with hydroxyapatite. Adhesive systems which attach to hydroxyapatite will form links with both enamel and dentine, although the bond to enamel for such materials is generally significantly stronger than the bond to dentine. Dentine offers the possibility of utilizing reactive groups, such as – NH which are present in dentine proteins, for achieving chemical union with adhesives. For adhesives of this type a significant bond strength may be demonstrated with dentine but no appreciable adhesion is observed with enamel.
In order to bridge the gap between the tooth surface and the resin-based restorative a series of difunctional chemical coupling agents were developed. Resin-based restorative materials were bonded to either dentine or enamel using coupling agents or adhesion promoters comprising difunctional molecules, one part of which enters into chemical union with the tooth surface whilst the other attaches to the resin, as illustrated in Fig. 23.6. The method of use was to apply the coupling agent to the clean, dry tooth surface followed by the resin filling material, normally a composite. The adhesion promoters had a general formula of the type shown in Fig. 23.7 where M represents a methacrylate group which eventually becomes bound to the resin by co-polymerisation, X represents a reactive group which interacts with the tooth surface and R is a linking and spacing group. Examples of such molecules are N-phenylglycine-glycidylmethacrylate, NPG-GMA (Fig. 23.8 and 23.9), 4-methacryloxyethyltrimelliticanhydride (4-META) (Fig. 23.10) and phosphate-methacrylates such as that shown in Fig. 23.11. In these, the mineral component of the tooth was thought to bind with the glycine, mellitic acid and phosphate groups respectively.
The coupling agents referred to above are all polymerizable monomers containing groups capable of interacting with the tooth surface. In the presence of the required initiators and activators the monomers are readily polymerised to form resins. Hence some of the products were supplied as two components, one containing a chemical activator (e.g. a tertiary amine), the other containing a polymerisation initiator (e.g. a peroxide). Over the years there has been a trend for chemically activated resin systems to be replaced by light-activated materials. As a result, some products are supplied as one component containing the adhesive and a light-sensitive polymerisation activator.
Fig. 23.6 Diagram illustrating the principle of bonding with a coupling agent. (a) Part of molecule which enters into bonding with restorative resin. (b) Part of molecule which enters into bonding with tooth surface.
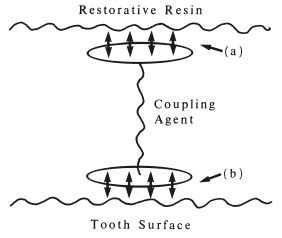
Fig. 23.7 General stru/>
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


