Implant‐Retained Restoration of the Craniofacial Patient
Robert Ferguson Wright, Glenn E. Minsley, and Sun‐Yung Bak
University of North Carolina, Chapel Hill School of Dentistry, Chapel Hill, North Carolina, USA
Facial defects from trauma, disease, or congenital abnormality have devastating psychosocial and functional effects on the patient. Missing anatomical portions of the maxillofacial and oral environment significantly affect esthetics, which makes it difficult for the patient to experience normal social interactions with family and friends. Surgical rehabilitation has been one of the options for restoring facial defects. However, sometimes “the treatment was worse than the cure,” resulting in facial disfigurement and oral dysfunction.1 Surgery is often limited by the availability of tissues and compromise of the tissue vascularity from previous radiation. Also, it may be advantageous to have exposure of facial defects so that oncologists can visually evaluate the surgical site for future recurrence.
Restoration of facial defects with facial prostheses has aided patients to experience less psychological trauma, as well as display a more normal esthetic appearance. The traditional means of retention of facial prostheses prior to craniofacial implants were through mechanical intrinsic undercuts and extrinsic adhesives.2 Often, mechanical retention was not adequate with the intrinsic undercuts, and patients experienced more embarrassment and psychosocial trauma when the prosthesis failed to adhere due to mobility of the adjacent tissues and failure of the adhesive itself. The adhesives used to hold the prosthesis in place damaged the prosthesis during maintenance, reducing the durability of the prosthesis and, often, it was difficult for the patient to place the prosthesis in the proper position every time.3 In addition, the adhesives were difficult to clean from the margins of the prosthesis and dermatitis was a common complication.4
In 1977, Per‐Ingvar Branemark and his colleagues placed the first craniofacial implant to retain a hearing aid. Craniofacial implants are extraoral endosseous implants inserted in the mastoid, orbital, and nasal regions, excluding the upper and lower jaws, which are parts of the craniofacial skeleton.5 The length of the implant is short since the thickness of the temporal bone is usually 4 mm. Craniofacial implants are 3–4 mm in length with a flange in the coronal aspect to provide primary stability and to prevent risk of penetration through thin bones, injuring other organs.6 Craniofacial implants enable the patients to position the facial prosthesis accurately in the same position every time the patients wear the prosthesis. Implants also extend the life of the prosthesis by not needing application of adhesives and solvents, which damage the prosthesis. In addition, they provide reliable retention to facial prostheses.7,8,9
The definition of “success rates” of implants is not uniform throughout the craniofacial area.10 “Implant success rate” and “implant survival rate” are not defined in the glossary of prosthodontic terms. Various authors have defined success and survival in different ways. Esposito et al.11 states that, for an implant to be successful, criteria such as function (ability to chew), tissue physiology (presence and maintenance of osseointegration, absence of pain and other pathological processes), and user satisfaction (esthetics and absence of discomfort) must be fulfilled. Otherwise, the implants are “surviving”, where implants are still in function but criteria for “success” or “failure” have not been met. Abu‐Serriah et al.12 agrees that “surviving implants are those that are still in function, but which have not been tested against certain criteria of success.” Abu‐Serriah questions whether success should be defined as a firm implant without clinical complications, such as poor tissue response, or if it should be defined as implants in functional service. In a 2008 clinical study of auricular prostheses, Wright et al.13 developed criteria of success. A systematic evaluation of the success of the implant prosthesis was developed. Criteria were established to organize reversible and irreversible factors with regard to both the integration of implants and qualities of the implant‐retained facial prostheses (Table 22.1). Implant factors examined included position, integration, and skin reactions. Prosthetic considerations included marginal fit, stability and function, symmetry/position, texture, color stability, and patient acceptance. Irreversible factors were those factors that could not be corrected, and therefore any one factor or problem would indicate a failure. Reversible factors required reassessment, analysis, and correction (Table 22.2).
Table 22.1 Criteria for success (Pt, patient; QoL, quality of life; Rxn, reaction).
Source: Wright 2008.13 Reproduced with permission of John Wiley & Sons.
| Implant | Prosthesis | |
| Reversible factors | Position (corrections angulated abutments, use of magnets instead of clips and bars) | Margins/marginal accuracy |
| Peri‐abutment skin Rxn: grades 0, 1, 2, and 3 | Color stability (extrinsic) | |
| Stability/functionality | ||
| Prosthesis, mobility | ||
| Symmetry/position | ||
| Pt acceptance/QOL | ||
| Irreversible factors | Integration vs. mobility/pathology | Color stability (intrinsic) |
| Position (cannot be corrected without compromising esthetics or functionality of prosthesis) | Contour/form | |
| Peri‐abutment skin Rxn; grade IV | Pt acceptance/QOL |
Table 22.2 Catergories I–V (Pt, patient; Rxn, reaction).
Source: Wright 2008.13 Reproduced with permission of John Wiley & Sons.
| Implant | CATEGORY | |||||
| I | II | III | IV | V | ||
| Reversible | Position (corrects: via angulated abutments, use of magnets instead of clips and bars) |
|||||
| Peri‐abutment skin Rxn: grade 0 | ||||||
| Peri‐abutment skin Rxn: grade 1 | ||||||
| Peri‐abutment skin Rxn: grade 2 | ||||||
| Peri‐abutment skin Rxn: grade 3 | ||||||
| Prosthesis | ||||||
| Margins/marginal accuracy | ||||||
| Color stability (extrinsic) | ||||||
| Stability/functionality | ||||||
| Prosthesis mobility | ||||||
| Symmetry position | ||||||
| Pt acceptance/quality of life | ||||||
| Irreversible | Implant | |||||
| Position (cannot be corrected without compromising esthetics or functionality of prosthesis) |
||||||
| Integration vs. mobility/pathology | ||||||
| Peri‐abutment skin Rxn: grade 4 | ||||||
| Prosthesis | ||||||
| Color stability (intrinsic) | ||||||
| Contour/form | ||||||
| Pt acceptance/quality of life | ||||||
Category I: ideal outcome Soft tissue and hard tissue condition healthy. Implants integrated, and prosthesis is esthetic and accepted by patient Grade 0 Holgers possible.
Category II: Minimal to no bone resorption and/or reversible soft tissue reaction up to grade 1 Holgers Prosthesis esthetically acceptable or may require minimal modification. Positioning or angulation of implants is not ideal but can be corrected.
Category III: soft tissue reaction requires prosthesis design or fabrication changes, and/or minor surgical intervention. Prosthesis requires moderate esthetic medification. Peri‐abutment skin reaction up to grade 2 Holgers. Requires correction that is difficult, but possible to achieve.
Category IV: prosthesis with reservation can be used, but retention or esthetics is compromised. Result is substandard. Prosthesis should be refabricated. Peri‐abutment skin reaction up to grade 3 Holgers.
Category V: prosthesis cannot be retained by implants due to lack of osseointegration or positioning that cannot be corrected. Peri‐abutment skin reaction up to grade 4 Holgers. All irreversible factors qualify Patient refuses to wear prosthesis.
Note: the most complex factor determines the overall classification for the case.
Earlier papers write about success rates,12,14,15 while later papers discuss survival rates.16,17 The survival rate is dependent on the time of follow‐up of the patient.17 The longer the time elapsed to evaluate the patient, the higher the failure rate.18 Therefore, the longer the follow‐up period, the shorter the survival rate. The survival rate of implants at 12 months at 100% is not necessarily indicative of success. Although there is discrepancy in the definition of rate of success or survival of implants, there is an agreement that the highest survival rate is among the auricular implants. There is a correlation between anatomical location of placement of craniofacial implants and clinical success.10,13 The highest success rate is found in the mastoid region and clinical study of 16 patients with implant‐retained auricular prostheses revealed a 100% success rate and the average follow‐up was 45 months.13
Roumanas et al.16 did a retrospective multicenter study of survival rates of osseointegrated implants used to retain orbital, nasal, and auricular prostheses in a 14‐year span. Out of 182 implants uncovered, 35 failed to integrate. The survival rate for all exposed implants was 80%. Auricular implants had a survival rate of 95–100%, while orbital implants had the lowest survival rate at 53%. Nasal implants had a 6‐year survival rate of 87%. A study done by Nishimura et al. for a 7‐year span (1987–1994) at UCLA found that there was 100% success rate of auricular implants, 71.4% for nasal implants, and 37.5% success rate of orbital implants.19,20,21 Wolfaardt’s et al.’s15 retrospective study of a survey of centers in Canada with a follow‐up period of 12–48 months found that the survival rates were 98.9% in the mastoid region and 96.6% in the orbital region. This relatively high survival rate for the orbit in the Canadian study may be explained by there being a relatively small number of patients compared to those receiving mastoid implants. In addition, the high survival rate could also be due to complex variables, such as a cohort of patients with good bone quality in the orbit. Other studies with 2 years or less of follow‐up time found higher survival rates of craniofacial implants. Scolozzi et al.22 reported implant survival in the mastoid region over 95%, nasal region 71–81%, and orbital region 35–91%. Wright et al.13 reported 100% survival rate in the mastoid region.
The auricular area has dense cortical bone, which provides good primary stability and vasculature for maintenance of the bone–implant interface.19 There is quite a disparity in the survival rate of implants depending on the anatomical location of the placement of implants in nasal defects. In implants restoring nasal prostheses, there is a lower success rate in the glabella compared to the anterior nasal floor. In their study, Nishimura et al. found 0% success rate in the glabella versus 88.1% success rate in the anterior nasal floor. The bone in the glabella site is dense, but the vascularity is poor with little marrow space, which may preclude remodeling necessary for osseointegration.10,20 In orbital defects, due to the curvature of the orbital rim, a large amount of bone needs to be removed in order to countersink the flange of the implant, which may create excessive thermal damage, compromising blood supply to the periosteum and causing bone necrosis.10 There is increased failure rate as more time passes, which is probably a result of the poor remodeling capacity of the bone–implant interface, lack of stabilizing bone volume in the frontal sinus, or decrease in vascular perfusion in these sites.10
Patients with facial defects from surgical treatment of disease often undergo radiotherapy. The effect of radiotherapy on tissues is very controversial. With radiation, some studies report progressive vascular damage while other studies find the vascular environment intact in high‐dose irradiation. Some believe that there is a period of time when there is actually growth of vascularity immediately after the radiation. Some experimental animal studies show an improved bone‐healing capacity 1 year after irradiation, and thus a long healing time has been recommended by some before implant placement.11 However, fibrosis and loss of vasculature has been observed to begin 6 months after irradiation in a human study, which appears to worsen over time. Thus, the recommendation by others has been to place implants within 6 months after irradiation.11
Even with conflicting reports of the effect of radiation on the vascular system in the irradiation area, there is agreement that there is a higher rate of loss of implants in irradiated sites compared to nonirradiated sites. Roumanas et al.14 found that the success rate of craniofacial implants for irradiated patients was 68.4% compared to 85.3% for nonirradiated patients. A stuby by Tolman et al.23 found the success rate to be 85% for irradiated patient versus 97% for nonirradiated patients. In a Swedish study, the success rate of craniofacial implants was 98.4% in nonirradiated sites compared to 57.9% in irradiated sites.24 Although the survival rates of implants are lower for patients receiving radiotherapy, radiotherapy is not a contraindication to placement of craniofacial implants.25 The factors that impact clinical success are the dose and fractionation of the radiation, and the anatomical site of placement of the implants.26
For radiation doses below 48 Gy, complications are rarely seen, whereas for doses above 64 Gy, there are many complications.11 Asikainen et al.27 did a study with mandibles of beagle dogs and found that there was a loss of all implants placed in mandibles irradiated with 60 Gy, few lost implants in mandibles irradiated with 50 Gy, and no loss of implants placed in mandibles irradiated with 40 Gy. In a meta‐analysis by Esposito et al.,11 there was a 2.6% loss of implants placed in jaws irradiated with less than 55 Gy while there was 10.1% loss of implants in jaws irradiated with 55 Gy or greater. The failure rate was 22.9% in the maxilla compared to 6.9% in the mandible in jaws irradiated with 55 Gy or greater. A review by Colella et al.28 noted no implant failures in patients who received less than 45 Gy.
In the irradiated temporal bone, auricular implants had 100% survival in studies by Parel et al.29 and Tolman et al.,30 while Granstrom et al.31 noted failures of 9% within a 5‐year follow‐up period. In a Swedish study, in the orbital sites, the success rate in the irradiated sites was 57% in a 5‐year span and 45.5% in a 12‐year span.24 With high doses of radiotherapy, success in the irradiated orbital sites ranges from 33% to 57%, possibly due to cellular and vascular changes in the irradiated bone. In nasal defects, there are too few studies to make any conclusive statements. Beumer et al.32 concluded from his retrospective analysis that osseointegration was impaired in bone for radiation doses in excess of 55 Gy and recommended hyperbaric oxygen therapy (HBO) to avoid risk of osteoradionecrosis if implant therapy was necessary.
HBO is a form of treatment where the patient is placed is a chamber with increased atmospheric pressure of oxygen. HBO therapy increases oxygen levels in the tissues, which increases capillary growth. It causes neovascularization, which causes epithelialization of soft tissue wounds.33 Adjunctive HBO therapy did not increase survival rate in the mandible, but appears to increase the survival rate in the maxilla as noted by Niimi et al.34,35 Also, Esposito et al.11 compiled a meta‐analysis and found that the implants placed in the irradiated maxilla with HBO had a 17.6% failure rate compared to a 31.7% failure rate without HBO. In the mandible, the failure rate was similar, at 4.3% with HBO and 5% without HBO.11
HBO may be beneficial as adjunctive treatment in irradiated tissue since it may decrease soft tissue complications and accelerate the healing process. Larsen et al.36 studied the placement of implants in the tibia of rabbits. There was 54% wound dehiscence in radiated tibias compared with 15% in nonirradiated tibias in rabbits not receiving HBO. In rabbits receiving HBO, there was 7% dehiscence in both the irradiated and nonirradiated tibias. In the animal model, the histomorphometric analysis illustrated that there was a significant improvement of integration between the implant and bone in HBO‐treated tibias for both irradiated and nonirradiated rabbits. With 16 weeks of healing, which is equivalent to 1 year, there was a greater percentage of integration than at 12 weeks. However, it is difficult to apply this animal model to humans. Granstrom et al.’s26 randomized study of patients in Gothenburg given HBO and patients not given HBO as control found that implant failure in patients given HBO was 2.6%, compared to 58% in patients without HBO in the maxilla and orbit. However, the studies of craniofacial implants placed in irradiated sites with and without HBO are too few in number, and the parameters of each study are so different in terms of length of the study and the amount of radiation delivered, that it is difficult to make any conclusive remarks.
Craniofacial implants must be carefully considered with appropriate presurgical planning. Patients must be aware of the failure rate and that prosthesis retention, esthetics, and the psychosocial benefits often outweigh the risks. With increase in time, there is decrease in survival rate of craniofacial implants, especially with radiotherapy where the side effects of avascularity and fibrosis increase with time. The use of craniofacial implants in maxillofacial prosthetics has certainly had a revolutionary impact on the treatment and rehabilitation of our patients when compared to the adhesive‐retained prostheses. The quality‐of‐life issues and advantages are numerous for bone‐anchored facial prosthetics.
Surgical phase
Presurgical planning
Presurgical planning for the placement of craniofacial implants begins with a diagnostic evaluation. The evaluation includes clinical examination of the defect site, as well as radiographic and/or stereolithographic model examination, to determine if there is sufficient bone thickness to accommodate placement of the craniofacial implants.37 As part of the diagnostic examination, a moulage should be made of the defect site. A diagnostic mock‐up of the final prosthesis should be performed to determine appropriate positioning of the craniofacial implants. A radiographic template is fabricated from the diagnostic mock‐up (Figure 22.1). The template is used to assist in assessing whether there is appropriate bone thickness for the planned craniofacial implants. The implants should be positioned in areas of the prosthesis that are of appropriate thickness to accept the abutment height and corresponding attachment components while maintaining the esthetics of the prosthesis. The average combined height for an abutment with a corresponding attachment could be in the range of 4–5 mm. Therefore, for example, in an auricular prosthesis, the implants should be positioned beneath the helical portion of the prosthesis, where the restorative material would be several millimeters greater than the 4–5 mm height range. This would adequately conceal the abutments and attachment system to provide proper esthetics for the prosthesis. Radiopaque markers are positioned within the diagnostic template where the craniofacial implants are to be placed and secured with an acceptable luting medium (Figure 22.2). The surgeon may want to select multiple sites for craniofacial implant placement, rather than a limited number, in case some sites reveal inadequate bone thickness on radiographic analysis. This could prevent any repeat of radiographic scanning with unnecessary exposure of the patient to radiation.
Figure 22.1 Radiographic template made from diagnostic mock‐up of final prosthesis.
Figure 22.2 Radiographic template in position on patient’s face. Radiographic markers (in this case, spherical lead shot seen as round gray balls) are embedded in the template in selected potential locations for craniofacial implant placement.
The patient is scheduled for computed tomography (CT) scan radiography of the defect site. At the time of the CT scan appointment, the radiographic template is positioned and secured within the defect and CT radiographs are made of the site. Examination of the radiographic sections will reveal the selected, proposed implant sites corresponding to the markers (Figure 22.3). The thickness of the bone can be measured to determine if adequate thickness exists for implant placement. Stereolithographic models can be employed to assist in this determination. A minimum of 3 mm of bone thickness is necessary to accept most craniofacial implants. However, it has been reported that endosseous dental implants can be substituted or combined with craniofacial implants in craniofacial regions where there is sufficient bone thickness to accept the minimum length standard endosseous dental implant.37,38 Appropriate sites for craniofacial implant placement are selected and noted on the template. The radiographic template can then be converted into a surgical template by removing the specific markers in the selected sites and drilling holes through the template to provide initial marking of the defect tissues for surgical implant placement.
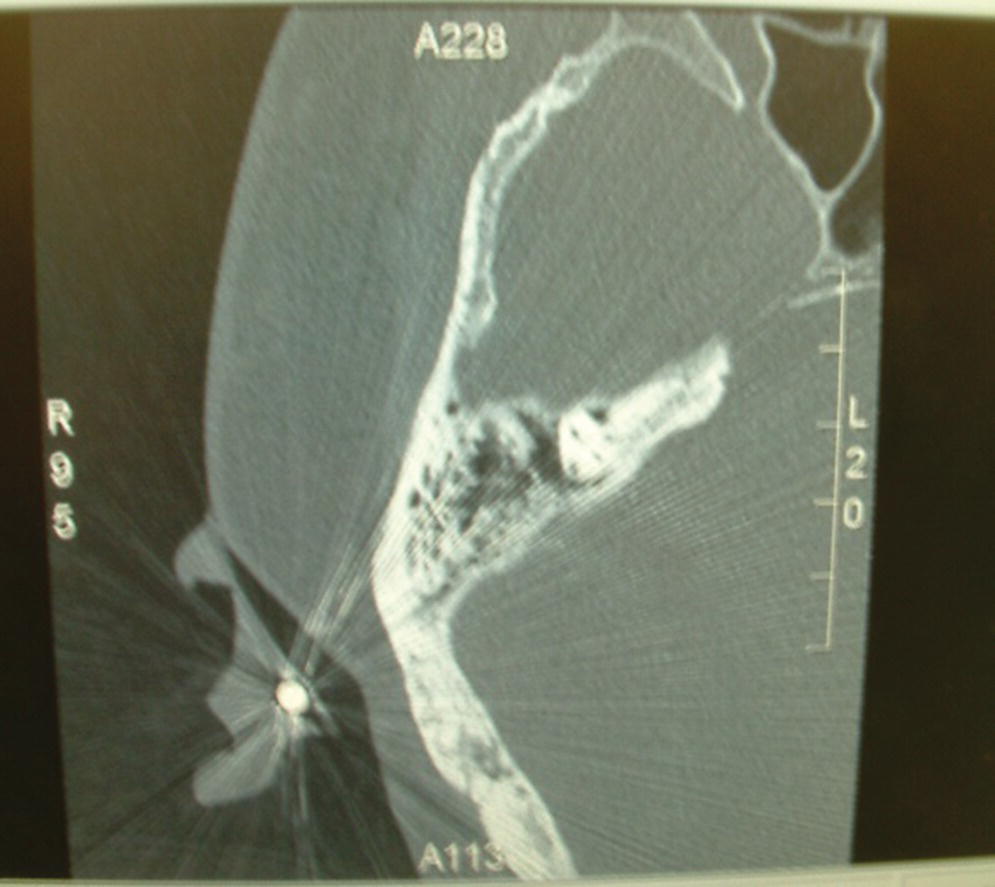
Figure 22.3 CT radiograph showing radiographic marker and associated thickness of bone adjacent to marker.
The literature reports recommended number of implants to be placed according to site.37 This is supported by data on success of osseointegration of craniofacial implants in each site as well as the size and weight of the prosthesis. When possible, the implants should be spread far enough apart to distribute the weight of the prosthesis proportionately. Two craniofacial implants are recommended for an auricular prosthesis. Branemark and his colleagues39 recommended positioning the two craniofacial implants based on number positions of a clock. Originally, for an auricular prosthesis on the right side of the head, the craniofacial implants would be placed at the 9 o’clock and 11 o’clock positions. For an auricular prosthesis on the left side of the head, it was suggested to place the craniofacial implants in the 1 o’clock and 3 o’clock positions. Later, the group revised the positions, moving the lower implant position to 8 o’clock for the right side and to 4 o’clock for the left side.40 Two to three implants can be used to retain a nasal prosthesis. The usual sites for craniofacial implants are the floor of the nose and the frontal bone. Three or more craniofacial implants are recommended for an orbital prosthesis, as the literature reported that the orbit has the lowest percentage of success of osseointegration.37 The main areas for craniofacial implant placement in the orbit are usually in the lateral wall and supraorbital rim regions of the orbit.
The craniofacial implant abutments should protrude through keratinized skin rather than nonkeratinized mucosa. The surgical placement of a split‐thickness skin graft may be necessary in the site where placement of the craniofacial implants would have the abutments protruding through mucosa.
Surgery
Surgical placement of the craniofacial implants should be performed in an operating room environment. The patient is anesthetized with general anesthesia or through conscious sedation. The patient is draped appropriately and the surgical site is isolated and prepared with a disinfecting solution such as betadine. The sterilized surgical template is positioned within the defect site. The holes designating the selected positions for the craniofacial implants are located in the surgical template. The skin and underlying bone are marked by inserting either a marker or a needle, coated with a dye or ink, through the holes in the template. If a marker is used to mark the skin, then a needle, coated with a dye or ink, should be used to pierce the skin until it contacts the underlying bone to leave a tattoo mark on the bone (Figure 22.4).
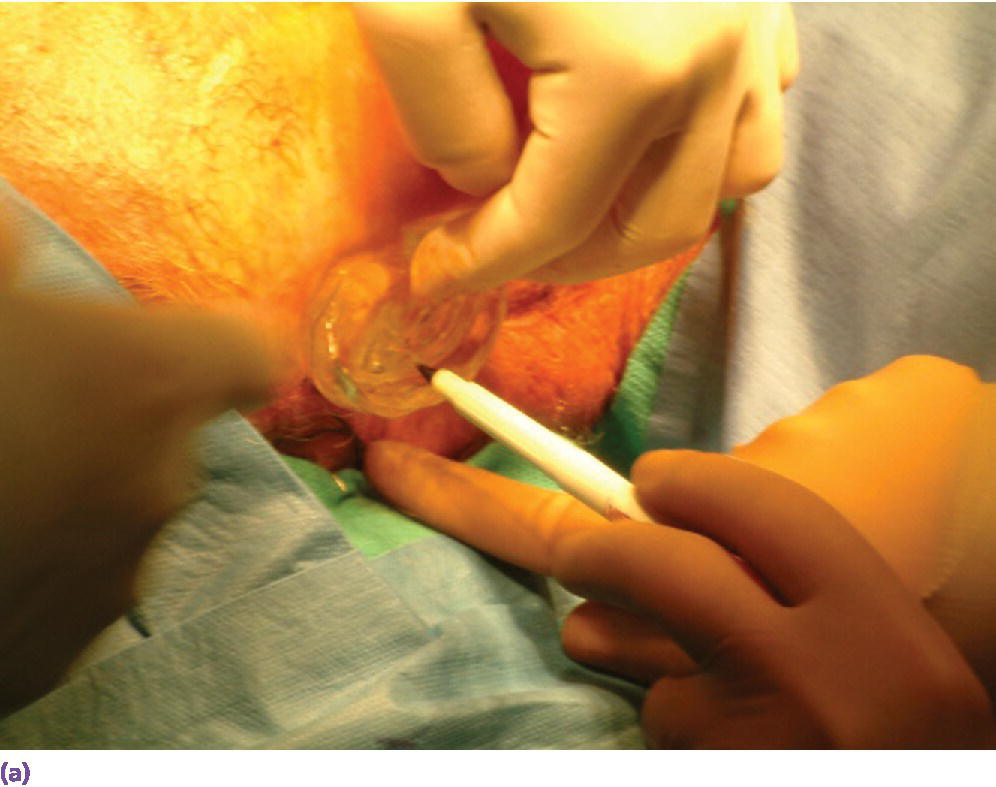
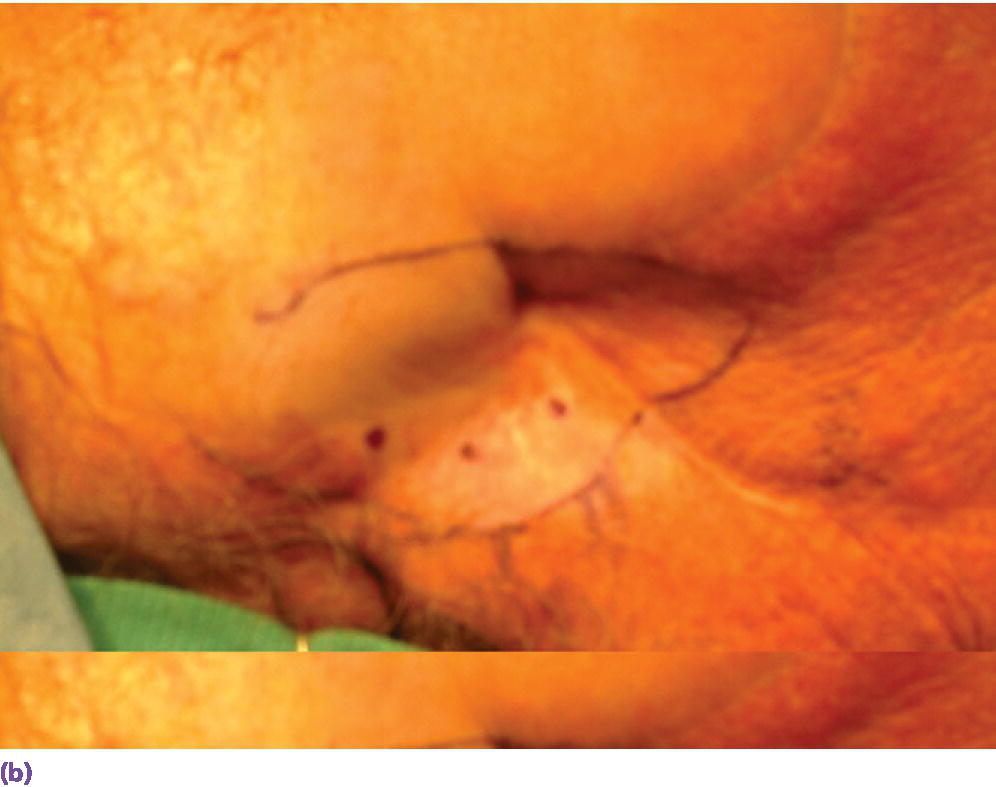
Figure 22.4 (a) Selected sites for craniofacial implants are marked with indelible dye or ink using a marker that penetrates holes drilled in template at the selected sites. (b) Marked sites for craniofacial implant placement. A needle coated with a dye or ink can then be passed through the skin at the indicated sites to tattoo the underlying bone. The curved line indicates the posterior border of the template for this case.
The surgical protocol, developed by Branemark, has been the hallmark of surgical placement of craniofacial implants.40 The original protocol is a two‐phase surgical procedure. The first surgical phase is the surgical placement of the craniofacial implants. Once the craniofacial implants are surgically implanted into the facial bone, the implants are covered by the overlying subcutaneous tissues and skin and osseointegration is allowed to proceed over a 3–4‐month period. No loading of the implants occurs during this time. Following this period, a second surgical procedure is performed to uncover the craniofacial implants. The final abutments are secured to the implants. The underlying subcutaneous tissues from the raised soft tissue flap are dissected and removed leaving a thin skin flap. Perforations are made through this skin flap in the areas where the abutments are to protrude through the flap. The skin flap is repositioned and sutured in place. Healing caps are secured to the abutments. A packing is placed over the skin flap and abutments to maintain pressure against the flap. This is to prevent hematoma formation and maintain contact of the skin with the underlying bone to allow for proper attachment during the healing period; this is usually 2–3 weeks. Prosthetic treatment can be initiated following this period.
There have been several changes in the design of the craniofacial implants over the years that have resulted in changes in the surgical protocol. The original Branemark craniofacial implants, which were threaded in design, required the use of a tap to create threads in the bone socket that was created for each implant. The implants were inserted by slowly screwing the implant into the tapped bone socket. Today, the new generation of the Branemark craniofacial implant, now marketed by Cochlear Corporation (Englewood, CO) under the name of Vistafix, are self‐tapping implants that do not require the creation of a pre‐tapped bone socket. This is true for some other craniofacial implant systems, such as the ITI craniofacial implant system marketed by the Straumann Corporation (Basel, Switzerland).41
The original Branemark craniofacial implants had a perforated metal collar around the head of the implant that overlaid onto the outer cortex of the bone to prevent potential iatrogenic damage to vital structures due to overseating of the implant. In addition, the head of the implant was an external hexagonal design. The newer generation of implants include craniofacial implants without the extended metal collar as well as an internal mortise design to the head of the implant.
Healing abutments have replaced the use of having to select a final abutment of appropriate length at the time of second‐stage surgery. This allows the restorative dentist or anaplastologist to carefully measure the thickness of the final skin flap and select a final abutment of correct length, following complete healing of the skin, rather than having to guess at the time of the second‐stage surgery.
In addition, the protocol has changed to allow for one‐stage surgical procedures.41 According to the Cochlear Corporation (Englewood, CO), in their Treatment and Surgery Guide manual for their Vistafix system, this protocol can be considered only for auricular prostheses when there is bone thickness greater than 3 mm with good quality to the bone.42
For the first stage of the standard two‐stage surgical approach, an incision is made remote to the designated areas for placement of the craniofacial implants. A full‐thickness flap is elevated to expose the underlying bone (Figure 22.5). The markings, previously tattooed with a needle coated with a dye or marker, are located on the cortex of the bone. Pilot drills of the appropriate depth, previously determined by the CT scans, are used to create the initial holes in the bone at the designated implant sites (Figure 22.6). Canon drills are then used to enlarge the sockets to the appropriate diameter and depth for the corresponding craniofacial implants. If the implant has a flange at the head, the drill is designed with a countersinking part to create a countersunk perimeter around the implant socket to accommodate the flange when the implant is inserted into the prepared socket (Figure 22.7). Each craniofacial implant is slowly screwed into position until fully seated in the prepared socket (Figure 22.8). Cover screws are inserted into each implant (Figure 22.9). The full‐thickness flap is repositioned and sutured into place (Figure 22.10). A surgical dressing is applied to secure the flap against the bone to insure attachment to the bone and to prevent hematoma formation beneath the flap (Figure 22.11). A period of 3–4 months is allowed for osseointegration of the implants.
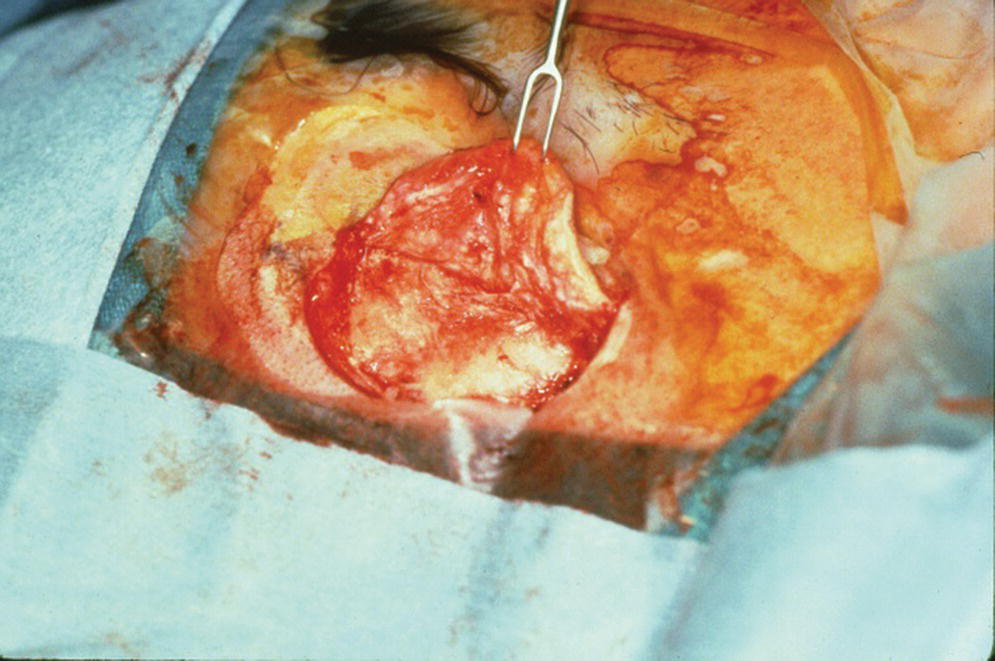
Figure 22.5 Elevation of full‐thickness flap.
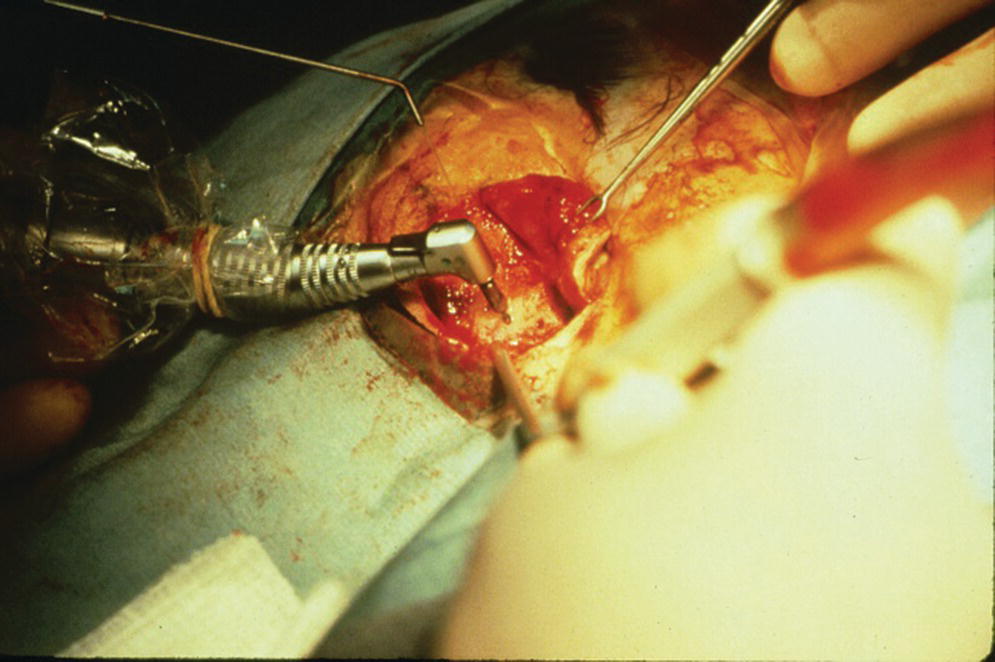
Figure 22.6 Pilot holes for craniofacial implant sockets are created with a pilot drill. Note that the pilot drill shaft has a narrow portion followed by a wider section. The wider section is at a selected distance from the drill bit (such as 3 mm or 4 mm) to define the specific depth of the pilot hole in accordance to the previously selected length of craniofacial implant for placement.
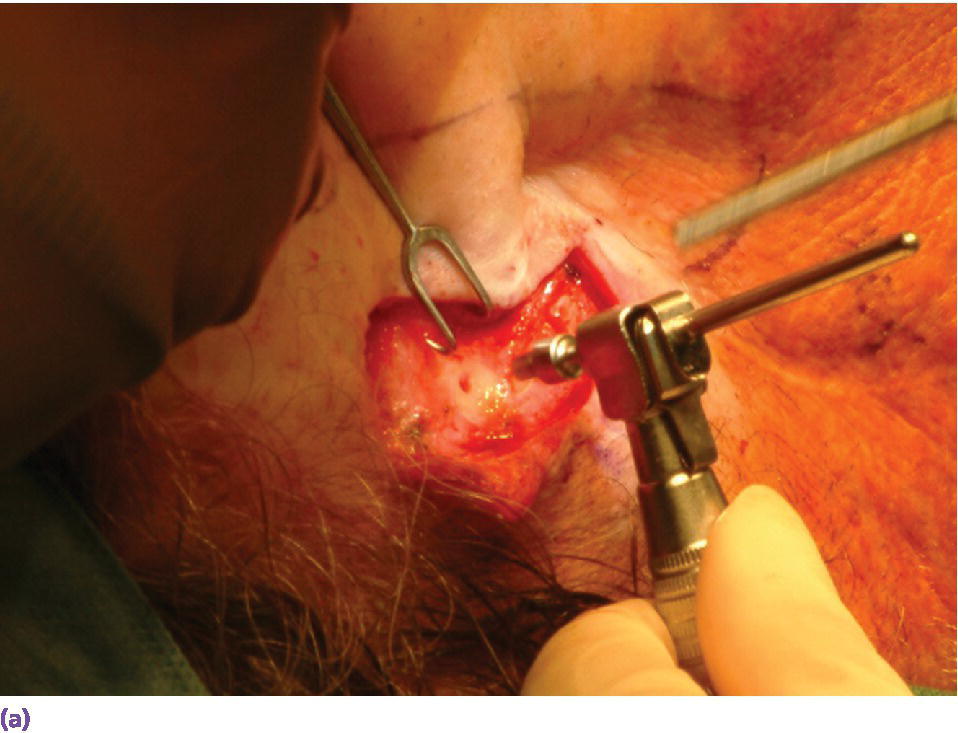
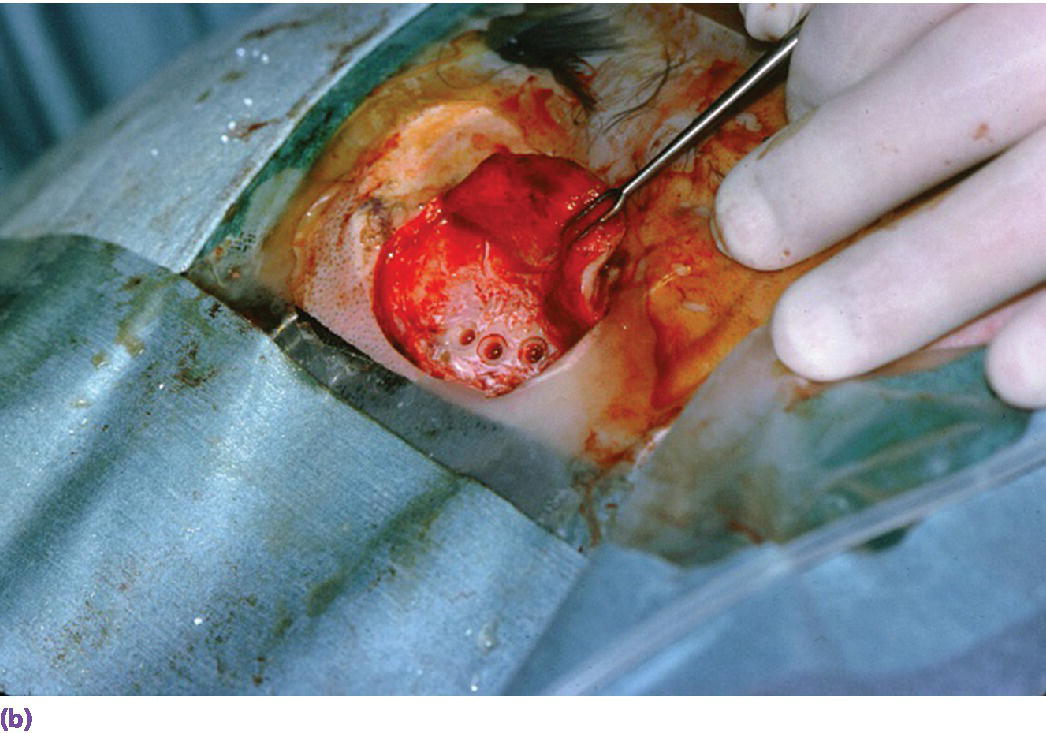
Figure 22.7 (a) Canon drill used to increase diameter of craniofacial implant socket to previously selected depth. If the craniofacial implant has a flanged head, the canon drill will also create the countersink surface to accommodate the flange. (b) Prepared craniofacial implant sockets with countersunk surfaces to accommodate flanges of the implants.
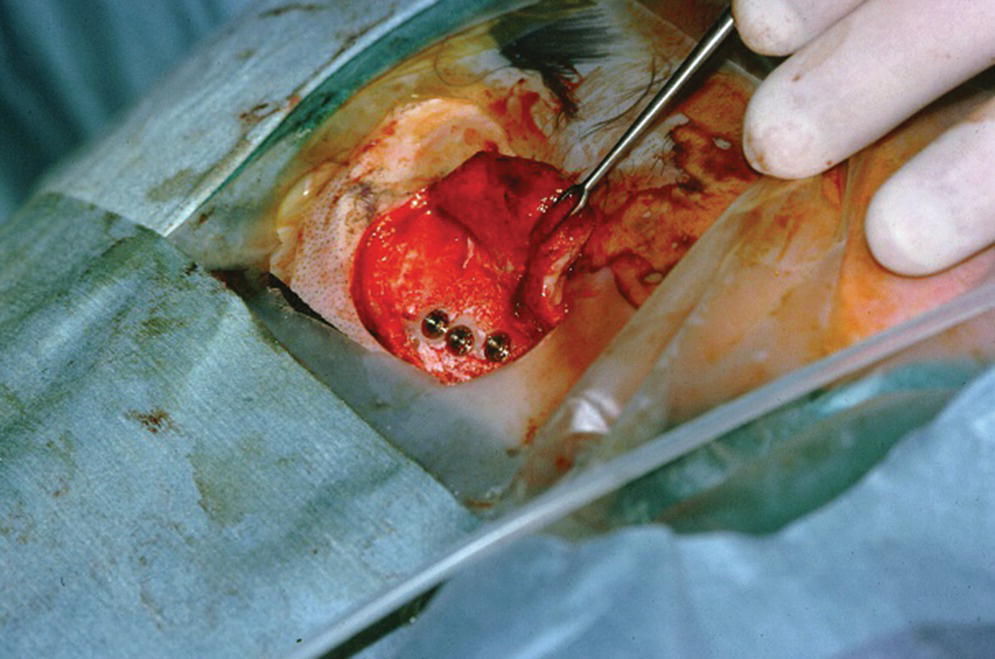
Figure 22.8 Craniofacial implants in position in respective sockets.
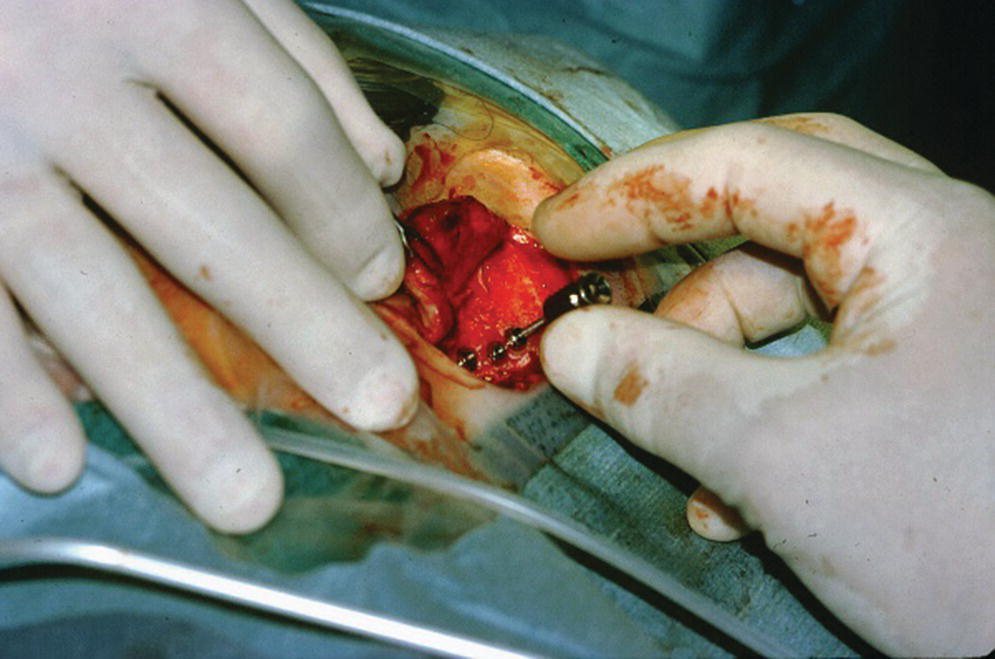
Figure 22.9 Cover screws are secured onto the craniofacial implants.
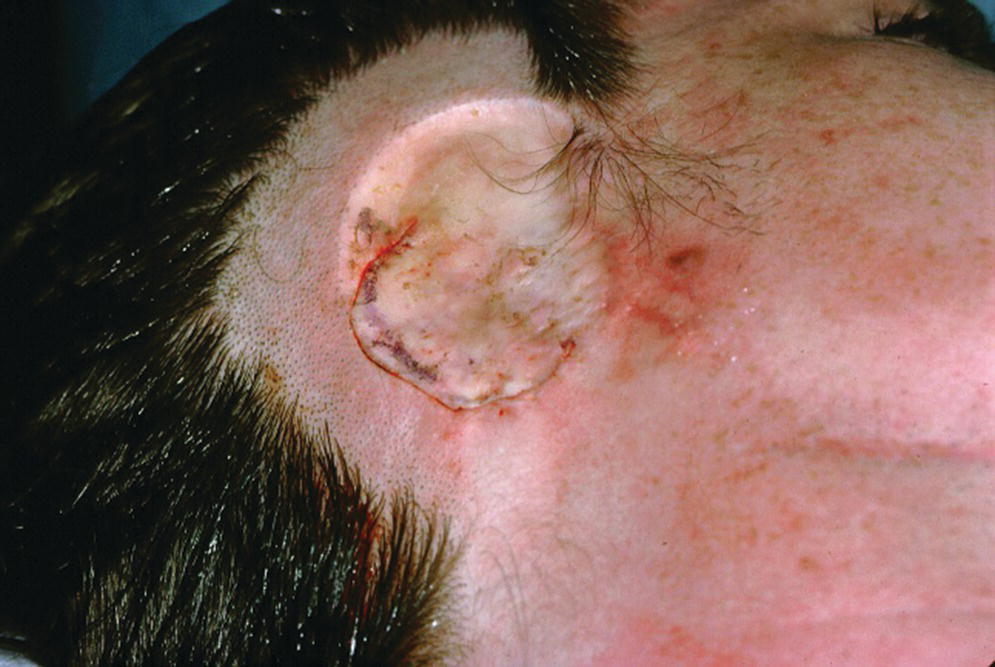
Figure 22.10 The full‐thickness flap is sutured into its original position.
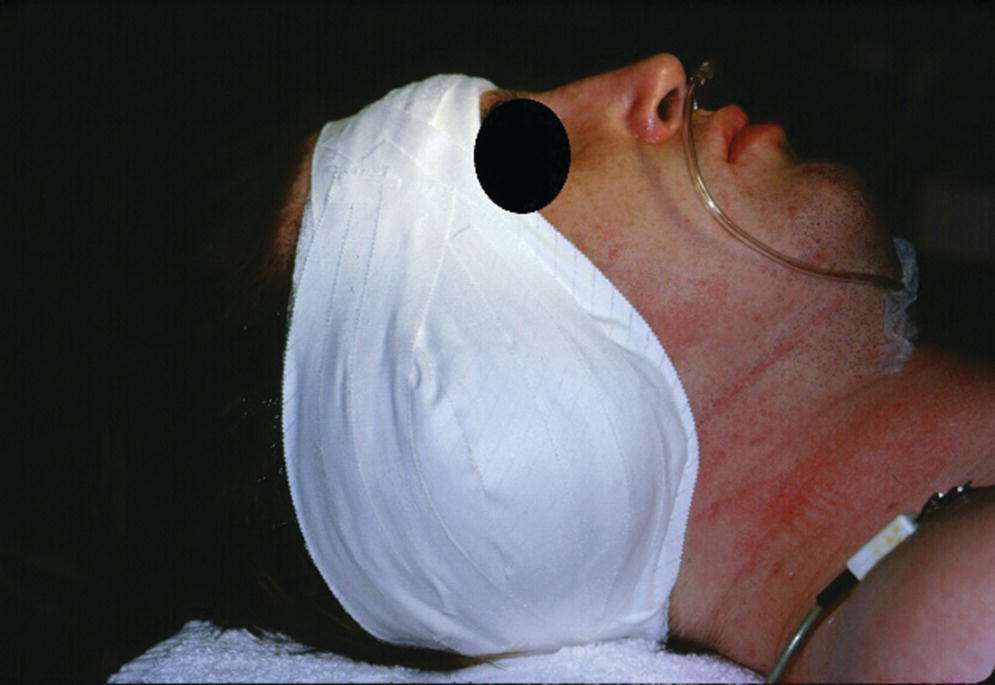
Figure 22.11 Surgical dressing is applied to maintain adaptation of the flap to the underlying bone for good attachment during the healing phase and to minimize hematoma formation.
The second‐stage surgery is performed following the 3–4‐month healing period. A repeat incision is made to elevate the full‐thickness flap to expose the underlying implants (Figure 22.12). Any overgrowth of bone over the head of the implant and cover screw is carefully removed without damaging the implant. The cover screws are removed from the implants. The implants should be tested to assure stability and rigidity as an indication of successful osseointegration. Any mobile implant should be deemed a failure and removed at this time.
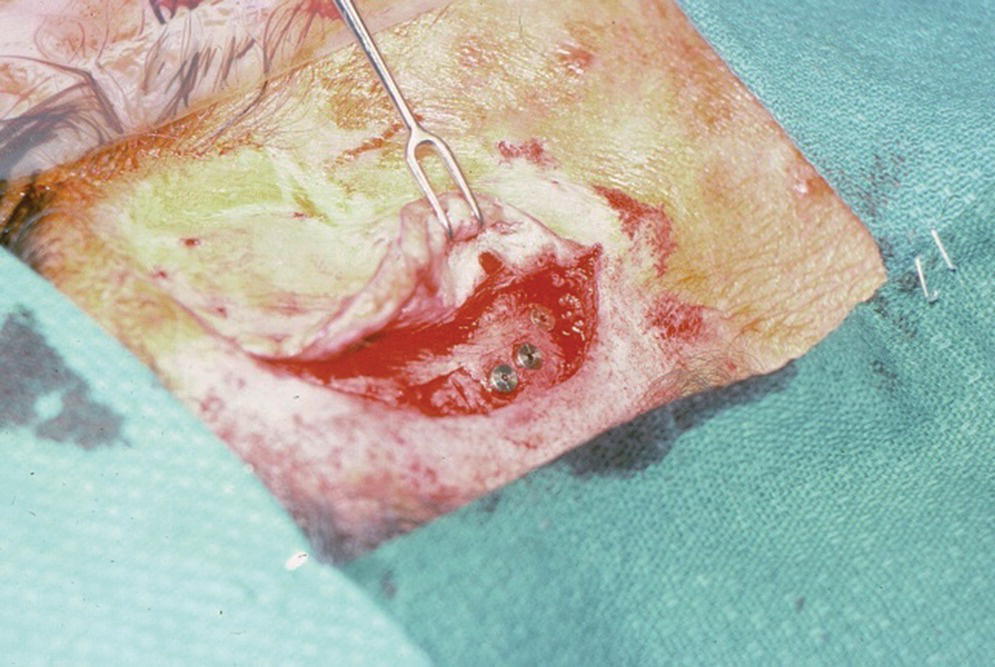
Figure 22.12 Full‐thickness flap is raised again during second‐stage surgery. The incision is made through the original incision site.
The full‐thickness flap is debulked of as much subcutaneous tissue as possible to create a thin skin flap (Figure 22.13). The flap is repositioned in place and perforations through the flap over the craniofacial implants are made with a punch (Figure 22.14). Either the actual abutments or healing abutments are then attached to the implants (Figure 22.15). The abutments should be of adequate length to extend transcutaneously at least 1 mm above the skin flap (Figure 22.16). The skin flap is sutured into position. If the final abutments are used, healing caps are secured onto the abutments. Another surgical dressing is applied to maintain adaptation of the flap to the underlying bone for good attachment during the healing phase (Figure 22.17). A period of 3–4 weeks is allowed to elapse for healing of the flap before initiation of prosthetic treatment.
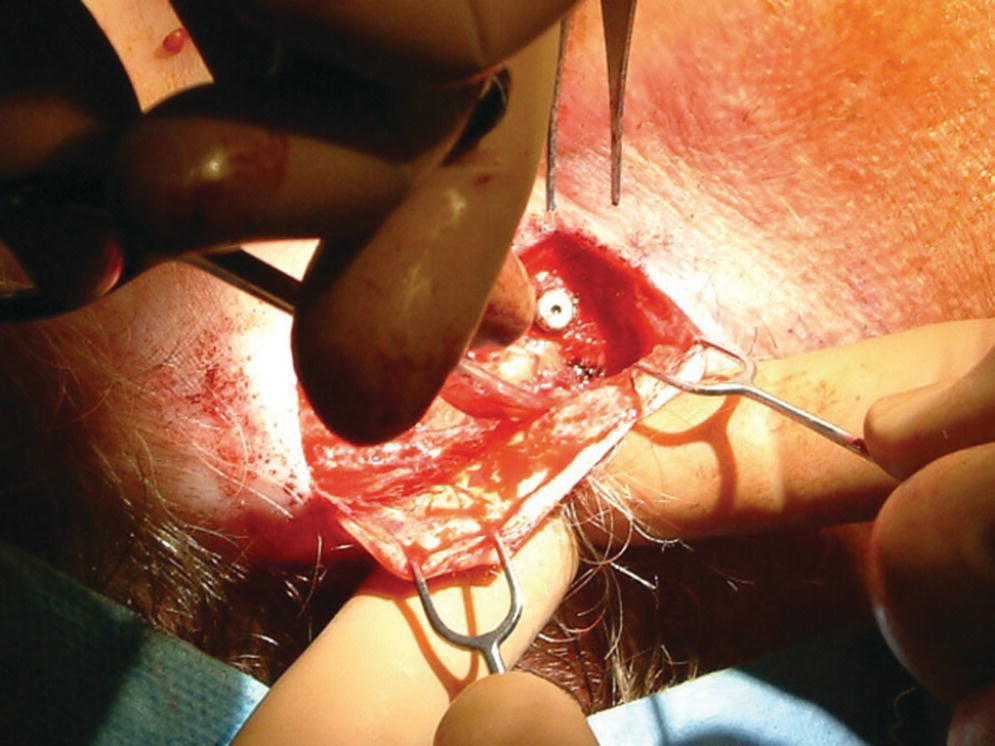
Figure 22.13 Debulking of the full‐thickness flap.
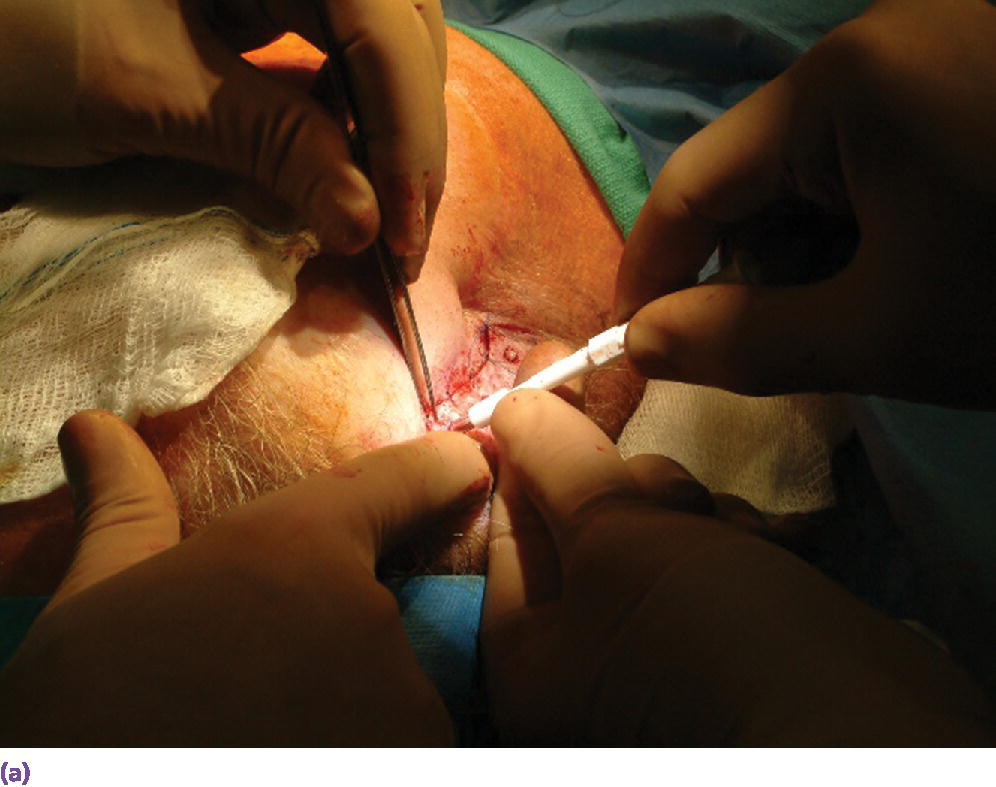
Figure 22.14 (a) The debulked flap is repositioned and holes are created through the flap over the underlying craniofacial implants using a surgical punch. (b) Punch holes through flap, exposing the underlying respective craniofacial implants.
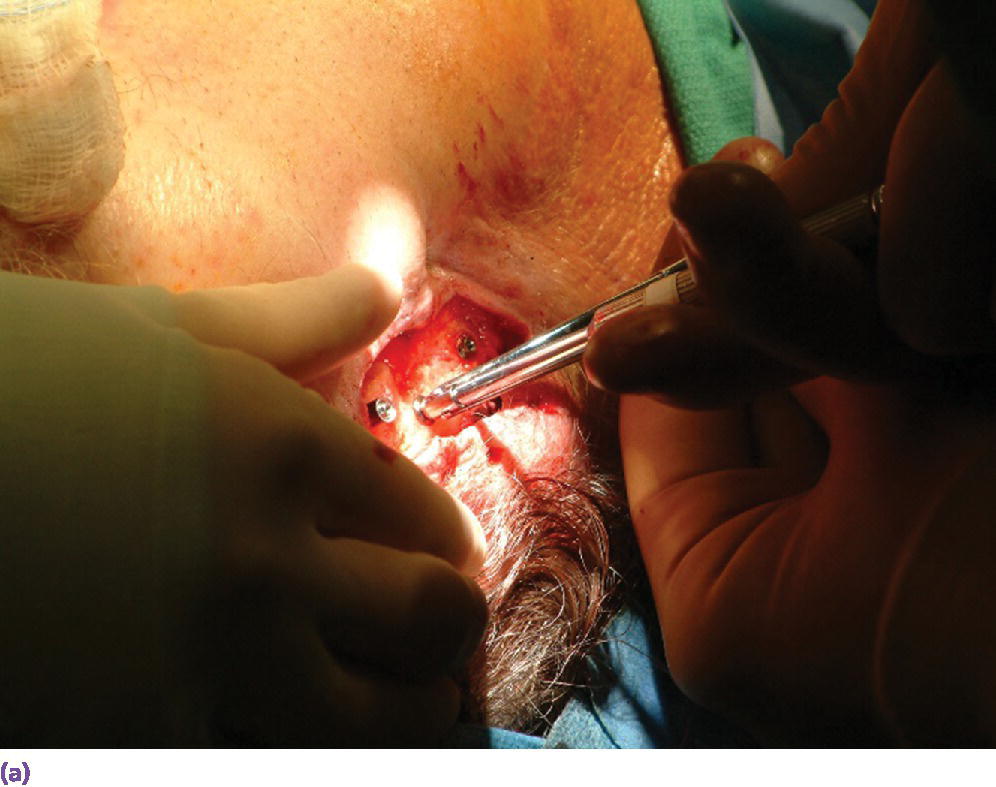
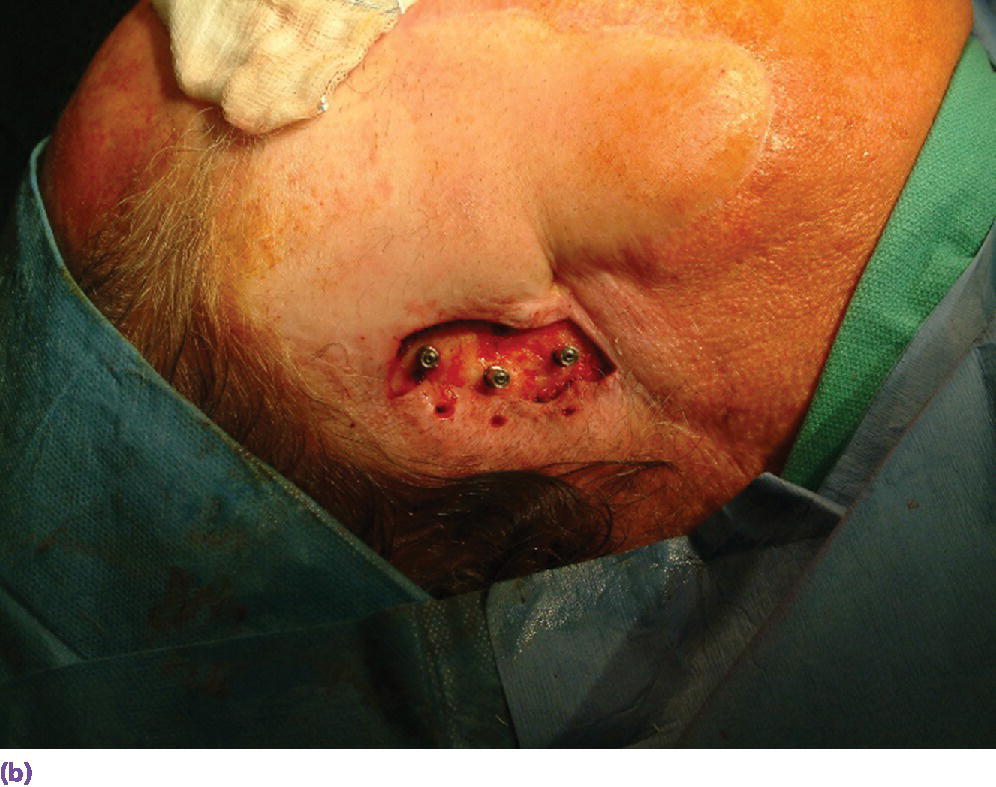
Figure 22.15 (a) Final abutments being secured to the craniofacial implants. (b) Final abutments secured to their respective craniofacial implants.
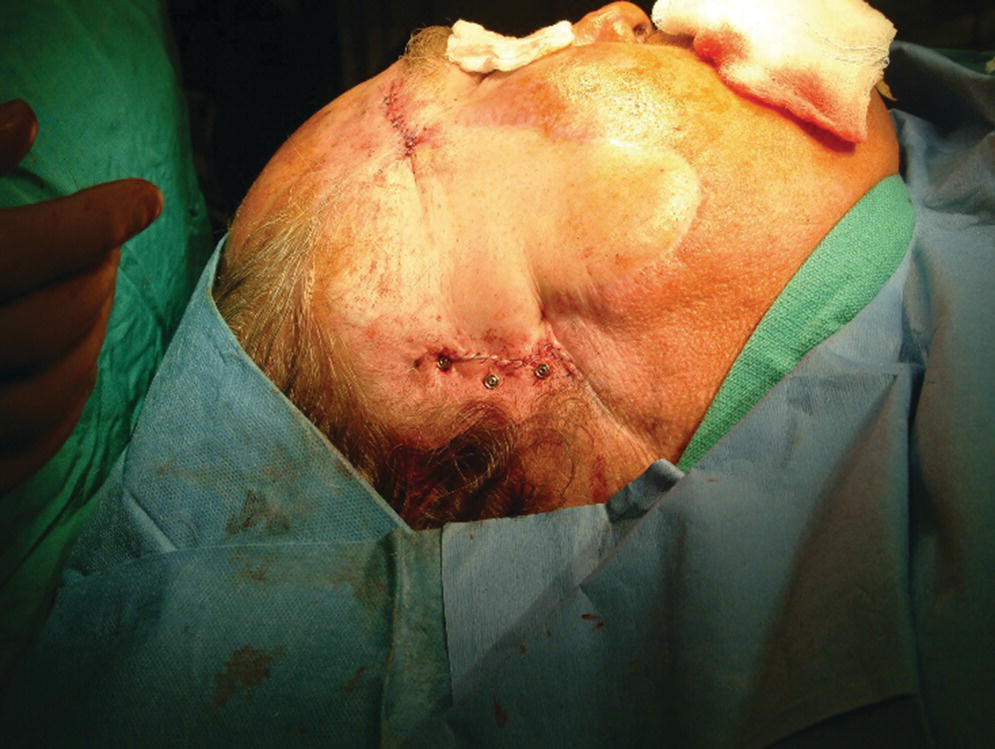
Figure 22.16 Skin flap repositioned and sutured in place. Note that the abutments protrude at least 1 mm through the skin.
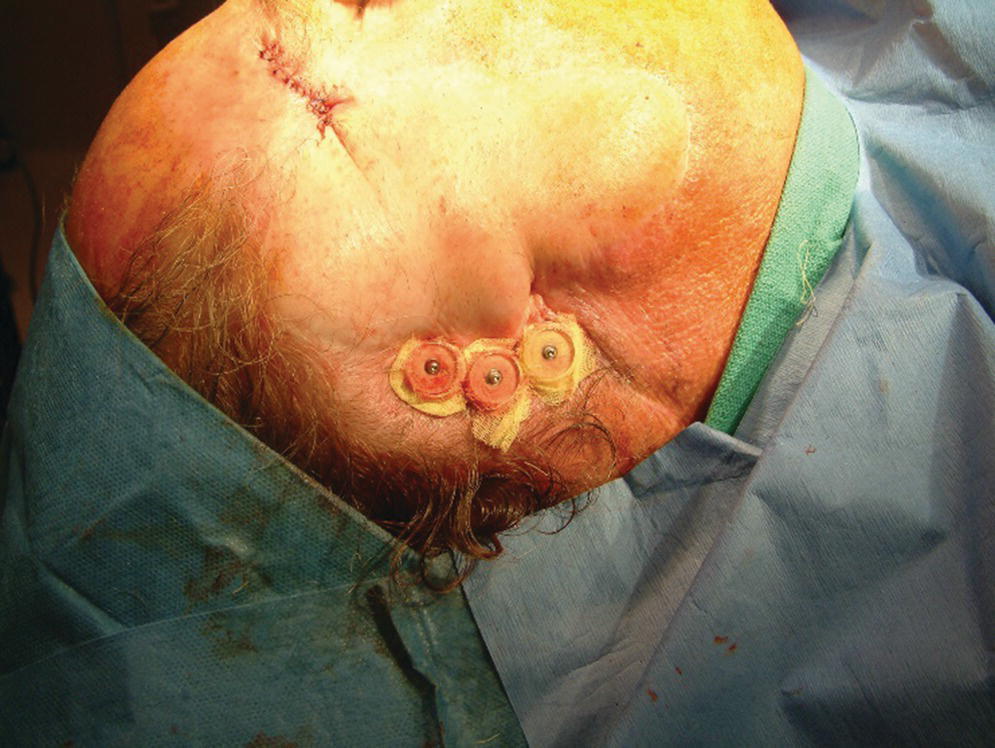
Figure 22.17 Healing caps and surgical dressing applied around the abutments and skin flap. In addition, a surgical packing will be placed to maintain light pressure against the flap to maintain position of the flap and prevent hematoma formation.
Nasal prostheses
The most common sites for placement of craniofacial implants for a nasal prosthesis are the floor of the nose and the glabella region (Figure 22.18). While the literature reports good success in osseointegration of craniofacial implants in the floor of the nose, there are unfavorable, albeit variable results, regarding success of osseointegration in the glabella region as has been alluded to in an earlier section.16,20,43 Roumanas et al.16 reported an overall 25% survival rate for craniofacial implants, ranging from 3–5 mm, in the glabella region. No time period before failure was reported in the article. Karakoca et al.43 reported a survival period of 30–33 months for two glabella‐placed craniofacial implants in their study. Based on these studies, it is recommended that the glabella region should be used as a secondary, alternate site, rather than a primary site for craniofacial implant placement in the nasal cavity.16,43 A radiographic template with radiopaque markers can be used to identify sites of sufficient bone thickness for placement of the implants such that the abutments and subsequent retentive components will not interfere with the esthetics of the prosthesis (Figure 22.19). CT radiographic scans with the template in position are made to ascertain appropriate sites of bone thickness (Figure 22.20). In addition, the use of three‐dimensional (3‐D) computer‐generated images or a stereolithographic generated model of the region can be helpful in making the final determination of appropriate implant sites (Figure 22.21).
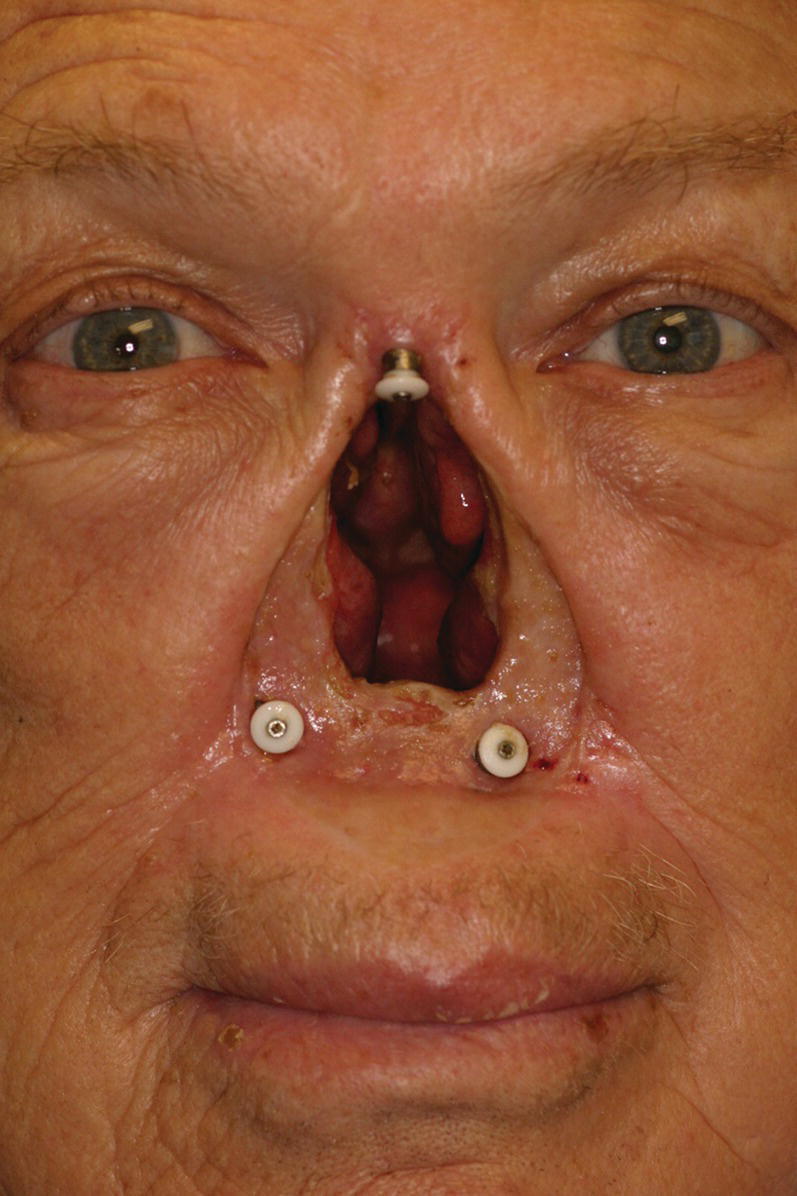
Figure 22.18 Craniofacial implants placed in floor of nose and in glabella region.
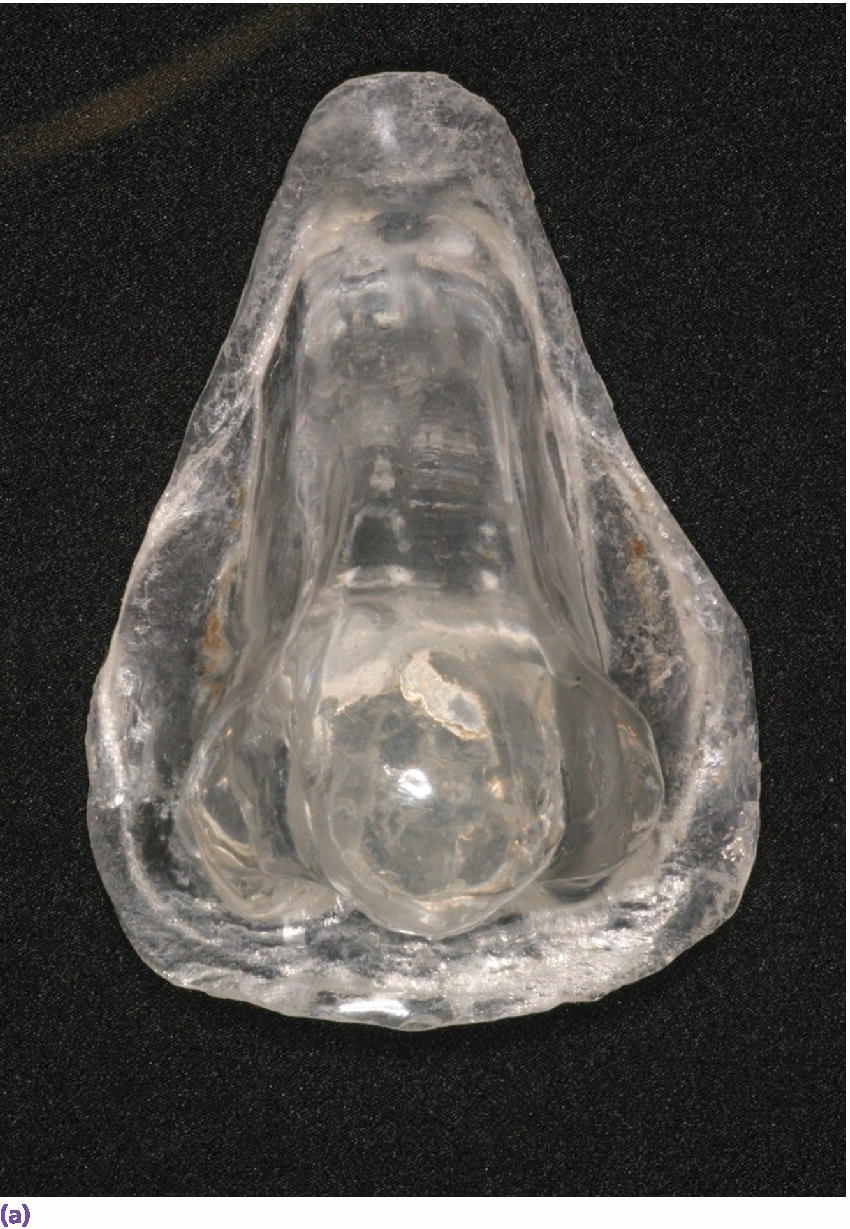
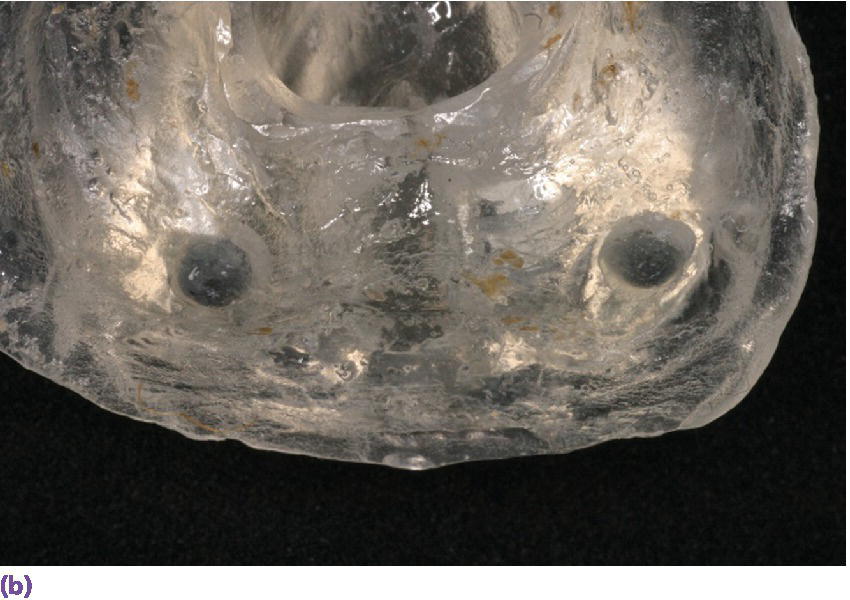
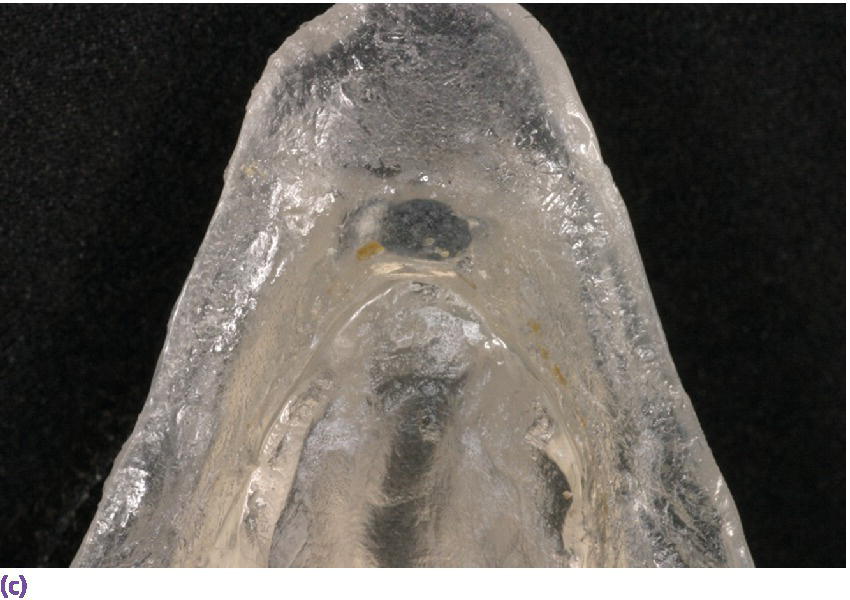
Figure 22.19 (a) Radiographic template. (b, c) Spherical lead shots, used as radiographic markers, embedded in template in potential locations for craniofacial implant placement.
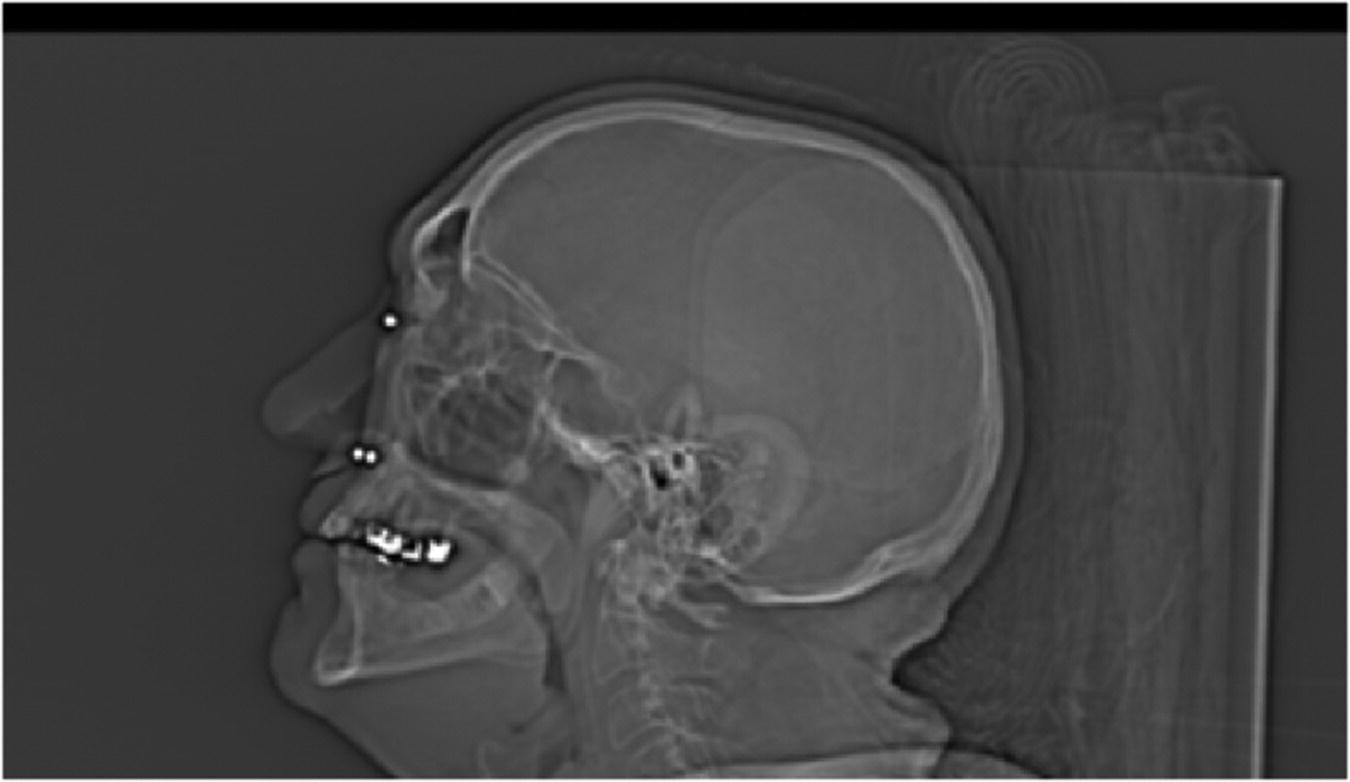
Figure 22.20 CT radiograph with radiographic template in position showing markers and thickness of adjacent bone.
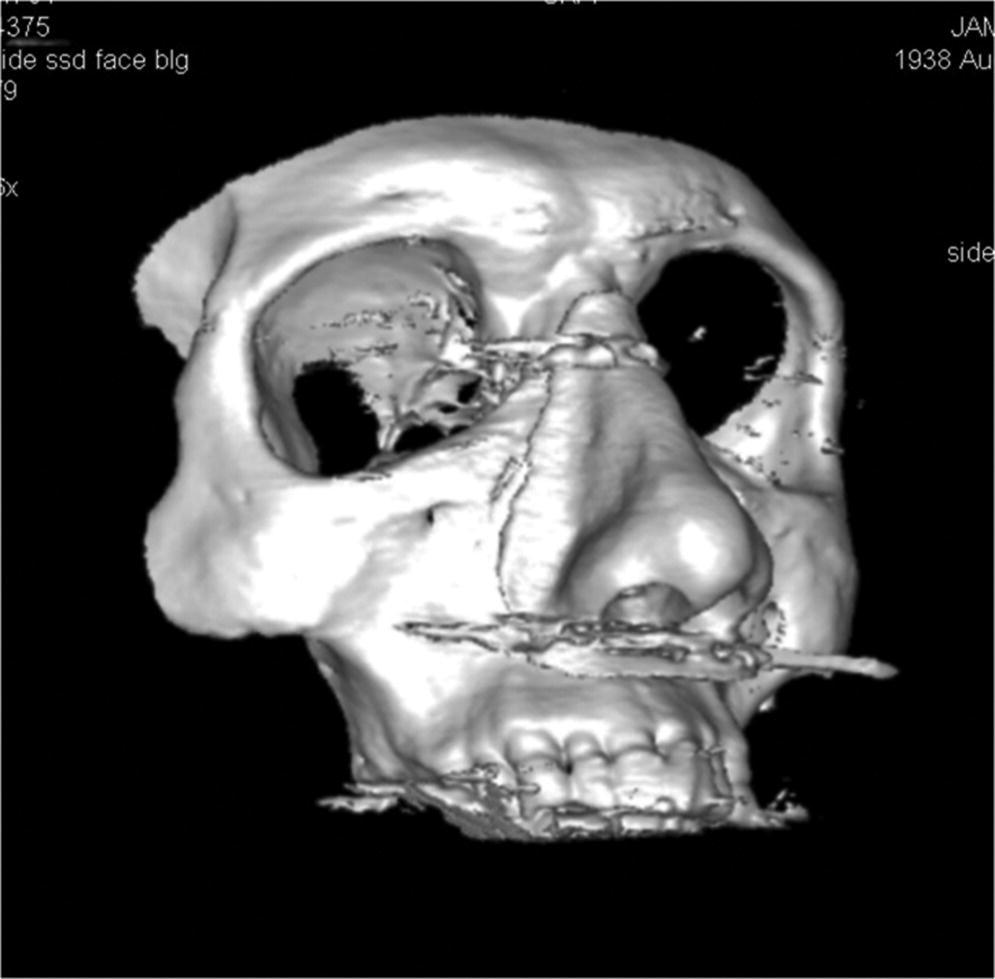
Figure 22.21 Three‐dimensional computer‐generated image of template and patient’s skull.
Once the sites have been determined, the radiographic template can be converted into a surgical template to guide the surgeon in the location and surgical placement of the craniofacial implants (Figure 22.22). After an appropriate time period to allow for osseointegration of the craniofacial implants, the definitive abutments can be attached to the implants. An attachment system can be designed to retain the nasal prosthesis. In many cases, this may be a framework, attached to the abutments, which employs a clip–bar retentive mechanism or incorporation of magnets for retention with the nasal prosthesis. Other retentive designs are the use of solitary abutments incorporating attachments such as magnets.
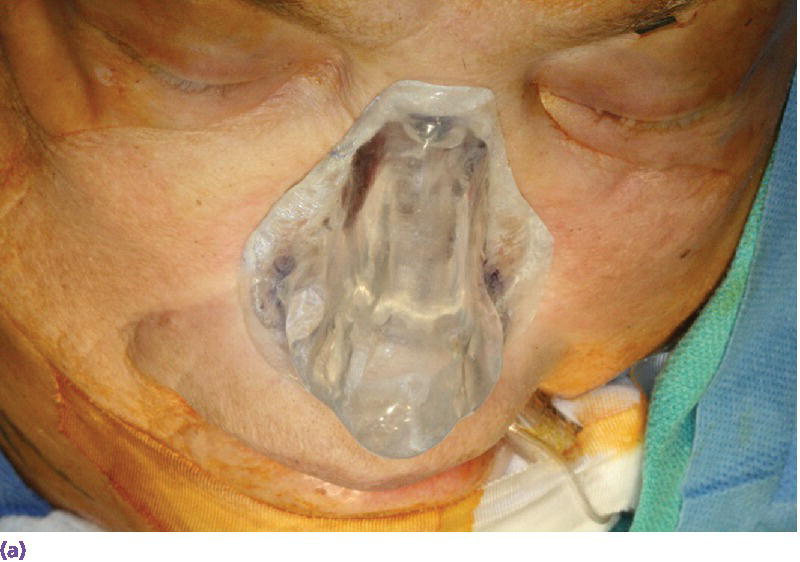
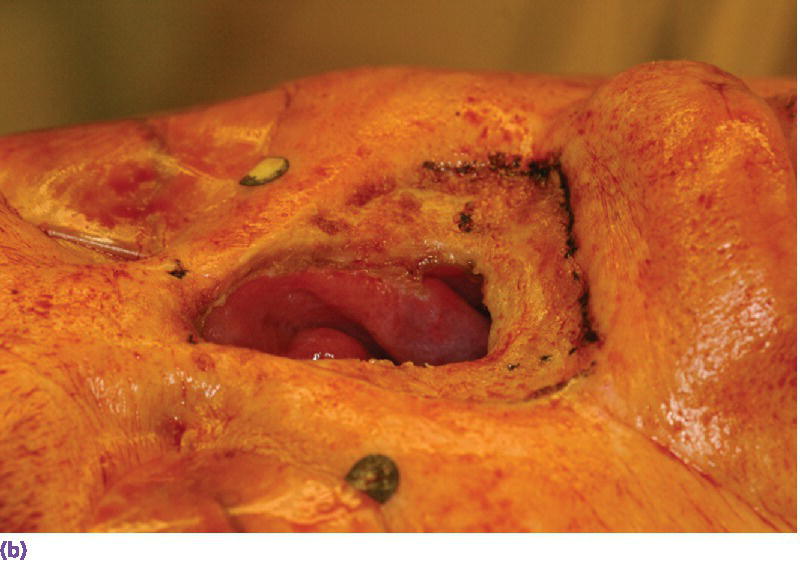
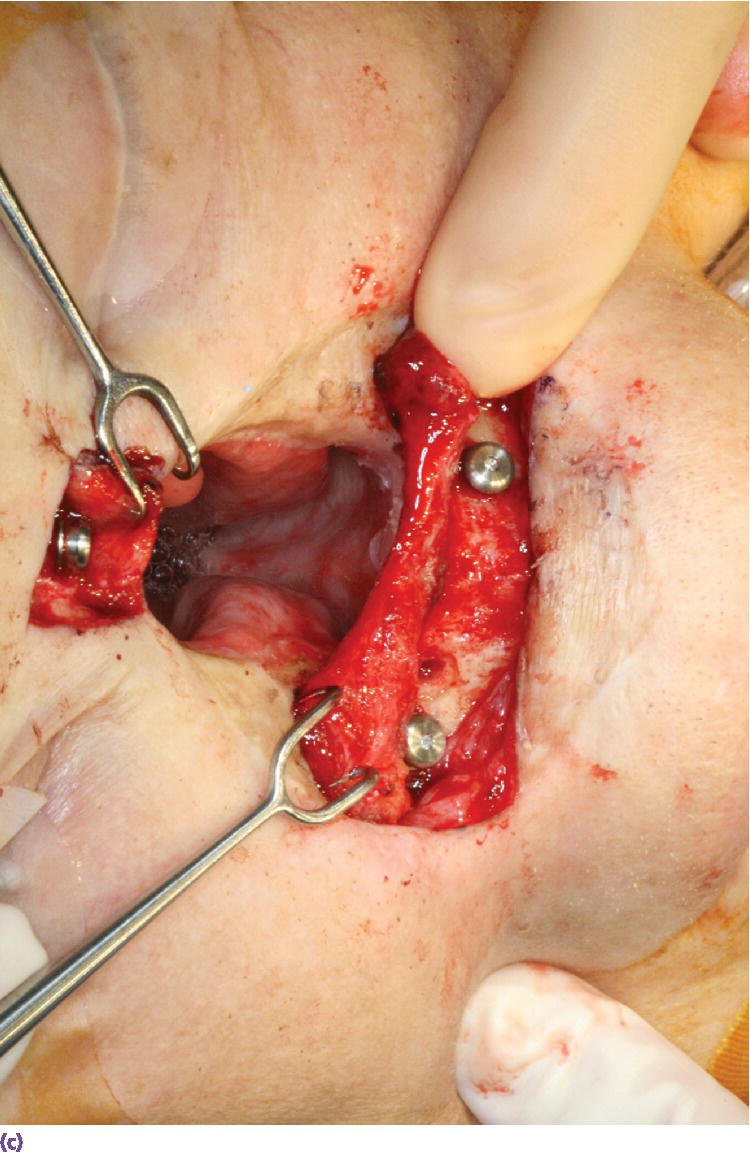
Figure 22.22 (a) Radiographic template converted to surgical template where holes were drilled through template in areas of radiographic markers. The surgical template is positioned on patient’s face and a marker with indelible dye or ink is passed through the holes in the template to mark the positions of the craniofacial implants on the patient’s skin. (b) Markings indicating the positions for the craniofacial implants in the floor of the nose and glabella regions. A needle coated with a dye or ink can be passed through the skin at these markings to tattoo the underlying bone. (c) Craniofacial implants placed in previously marked sites.
If a framework is to be used as part of the retentive mechanism, a moulage of the nasal defect and surrounding facial regions is made. Impression copings are attached to the abutments prior to making of the moulage. The copings can be splinted together to form a stable unit that will be incorporated in the moulage (Figure 22.23). The moulage is then made of the region (Figure 22.24). Abutment analogs are attached to the impression copings and the moulage is poured with dental stone to produce the master cast.
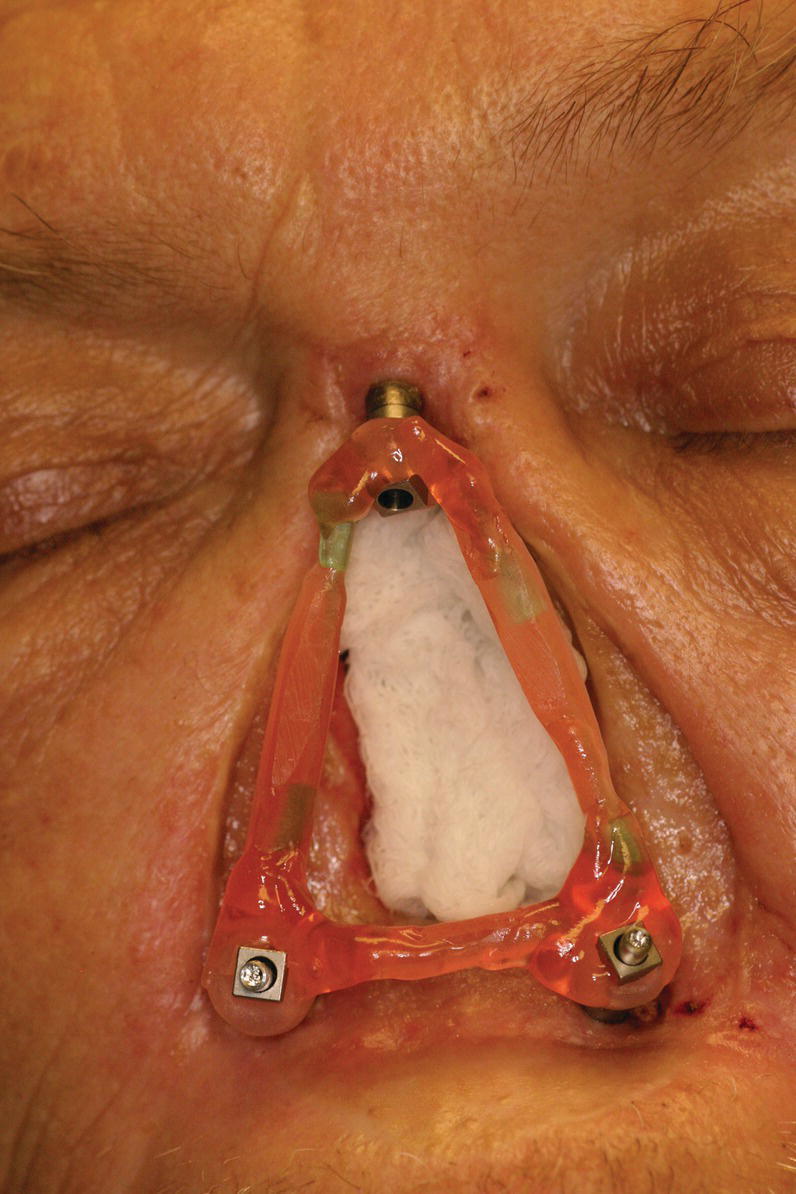
Figure 22.23 Splinted impression copings attached to craniofacial implant abutments.
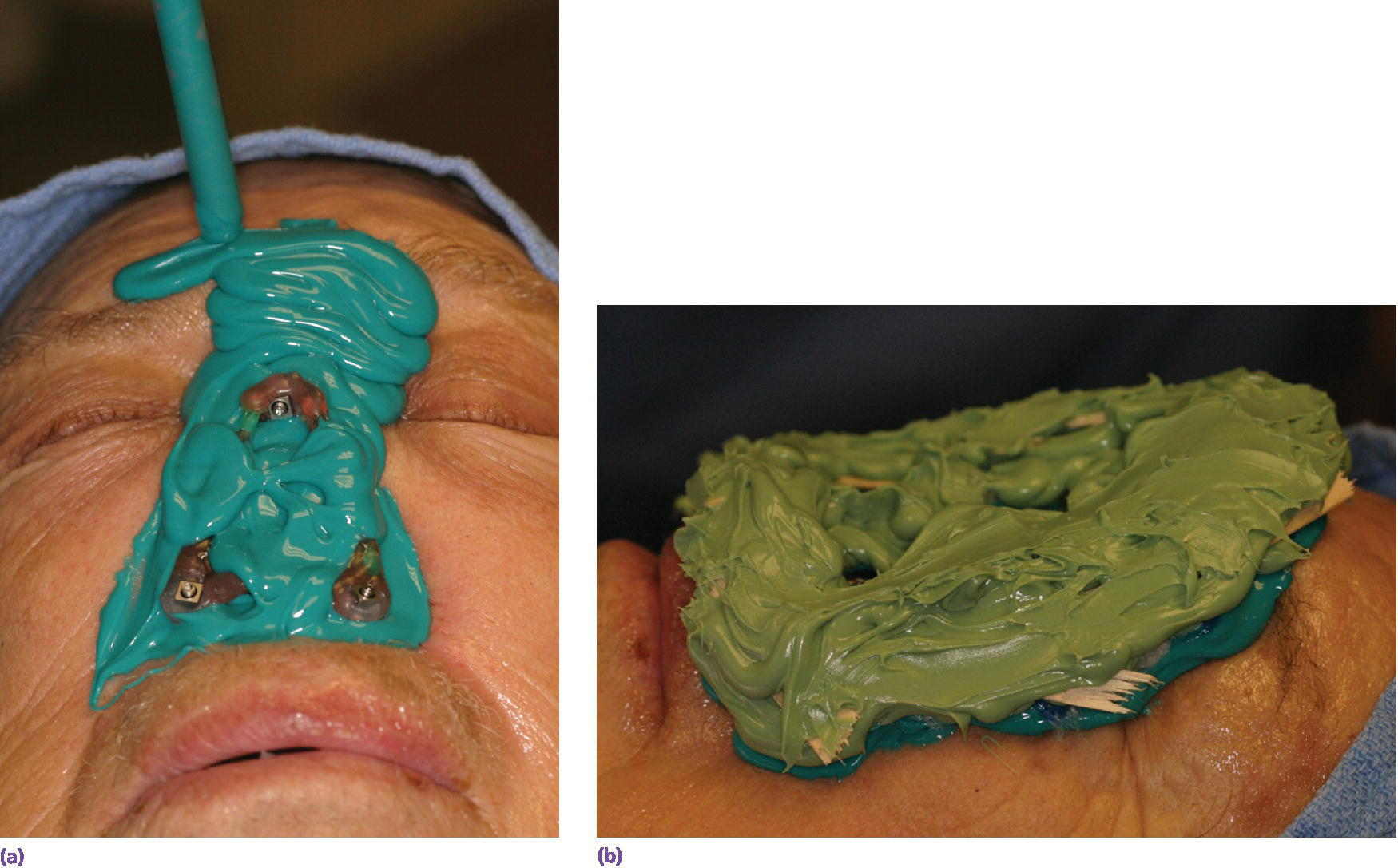
Figure 22.24 (a, b) Moulage made using low‐viscosity vinylpolysiloxane impression material supported by high‐viscosity vinylpolysiloxane impression material with pieces of tongue blades embedded within the high‐viscosity material to assist in providing a rigid “tray” to support the low‐viscosity impression material.
The framework can be fabricated through a conventional method of casting in a metal alloy from a wax pattern, or soldering prefabricated metal bars to metal alloy copings. More recently, computer‐assisted design/computer‐assisted milled (CAD/CAM) technology is being employed in the fabrication of such frameworks (Figure 22.25). The framework should be attached to the abutments in the defect on the patient and evaluated to insure that it fits passively.
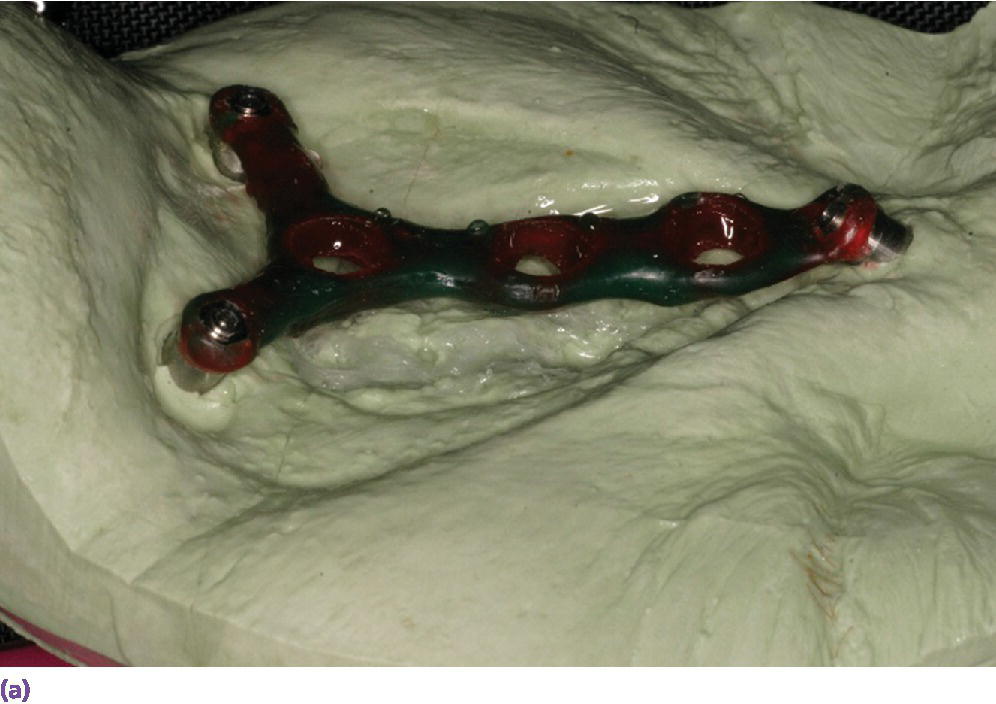
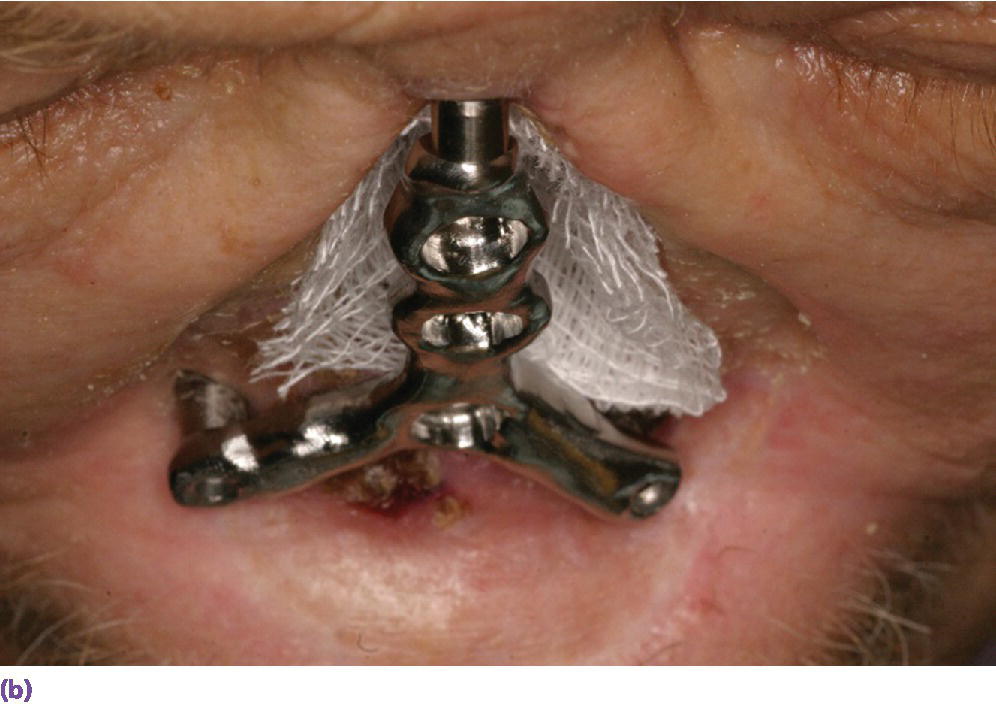
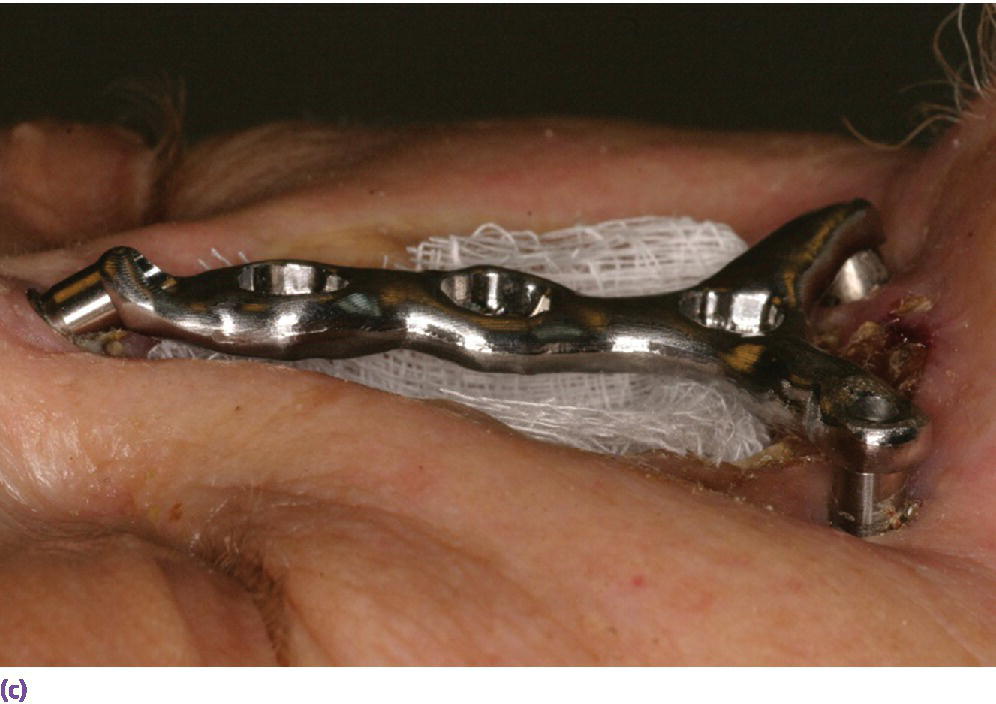
Figure 22.25 (a) Master cast with incorporated abutment analogs with a pattern for framework made from acrylic resin and wax. Framework will incorporate three cobalt–samarium magnets to provide retention of the nasal prosthesis to the CAD/CAM titanium alloy framework. (b) CAD/CAM titanium alloy framework made via copy mill process of framework pattern. (c) Sagittal view of CAD/CAM titanium alloy framework.
A superstructure can then be fabricated to fit over the framework. This is usually made in acrylic resin or similar material. The superstructure will incorporate the reciprocal retentive components that will join to those in the framework to provide retention for the prosthesis (Figure 22.26). The superstructure can be made hollow to allow for patency of the nasal airway in the prosthesis. This superstructure eventually becomes incorporated into the final prosthesis.
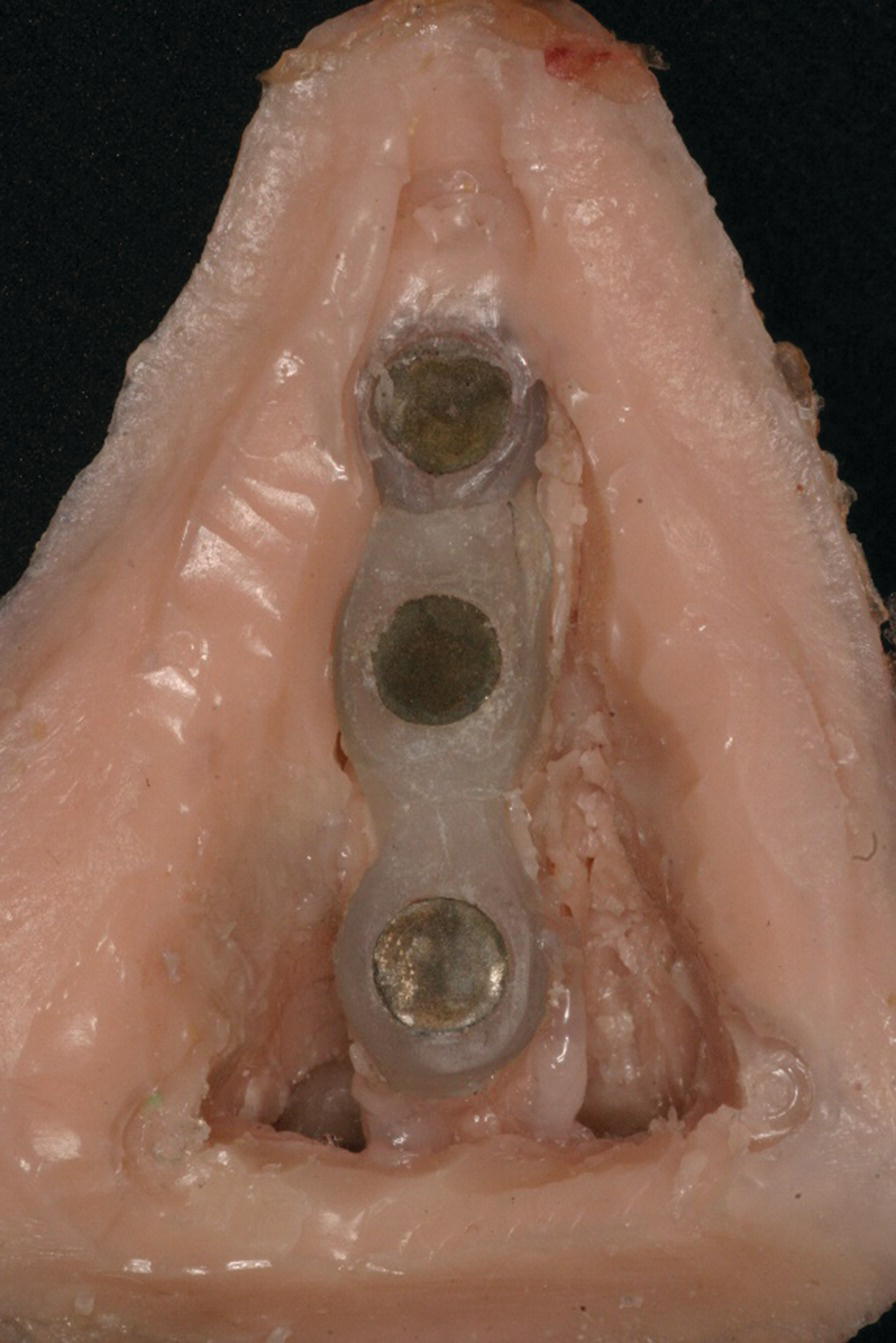
Figure 22.26 Acrylic resin superstructure framework, housing a matching set of cobalt–samarium magnets, embedded within the final prosthesis.
The contours for the nasal prosthesis are created in wax or in clay over the superstructure. The entire pattern is positioned on the patient’s face to evaluate for accuracy of fit, anatomic detail, and marginal integrity with the adjacent skin. Further refinements are made until the final results are achieved in the pattern (Figure 22.27). The pattern, along with the master cast, are invested and the wax or clay is thoroughly removed from the mould. A material, such as silicone, is used for the outer overlay of the prosthesis. Color is incorporated intrinsically into the silicone using artist oils and/or dry earth pigments to create the intrinsic skin tones. The silicone is packed, under pressure, in the mold and processed to a complete curing of the material. The final prosthesis is then removed from the mold. The prosthesis is positioned on the patient. Margins are trimmed accordingly for proper marginal adaptation. Extrinsic coloration is performed to create the final surface characteristics on the prosthesis that match and blend with the adjacent skin of the patient’s face (Figure 22.28).
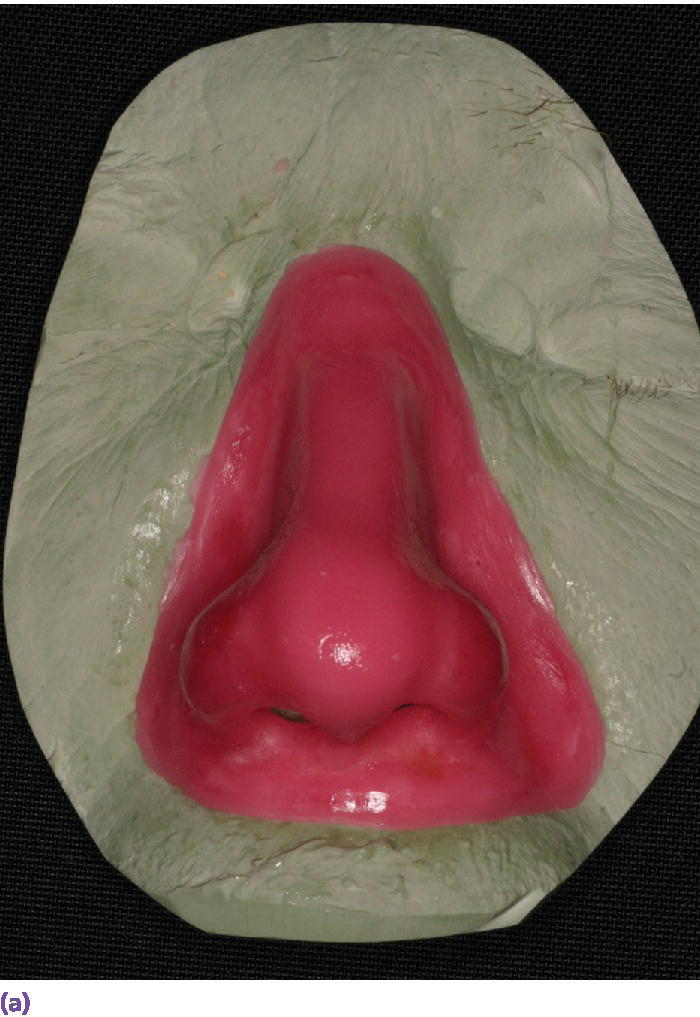
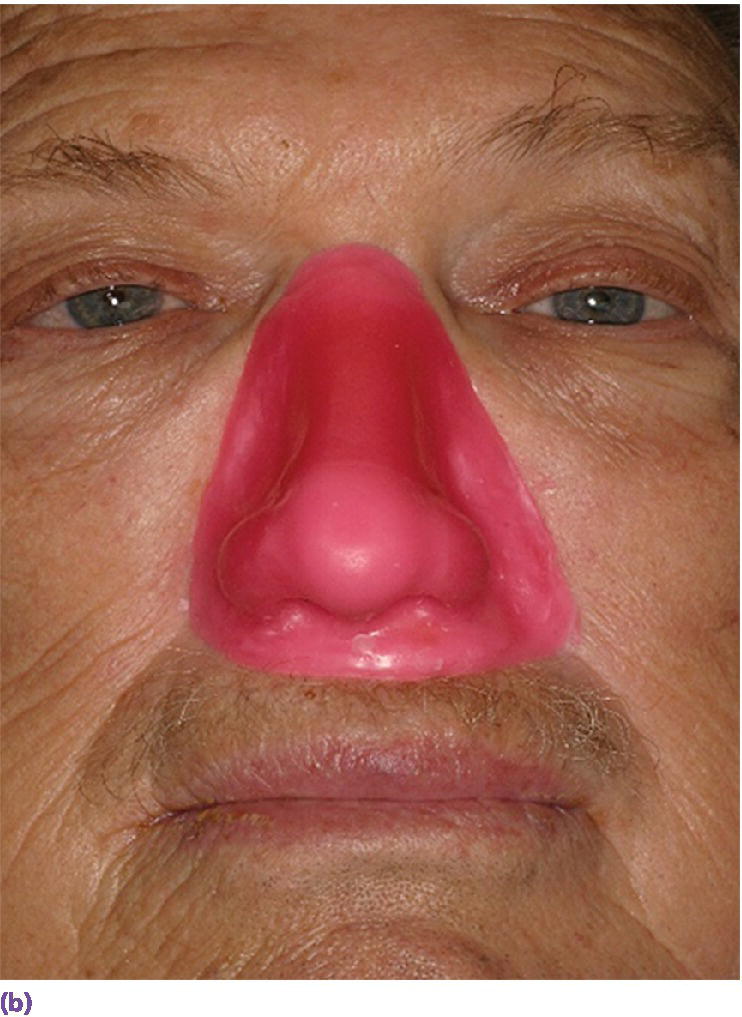
Figure 22.27 (a) Final wax pattern for nasal prosthesis on master cast. The wax pattern incorporates the acrylic resin superstructure framework. (b) Final wax pattern positioned on patient’s face to check for accuracy of contours and marginal integrity.
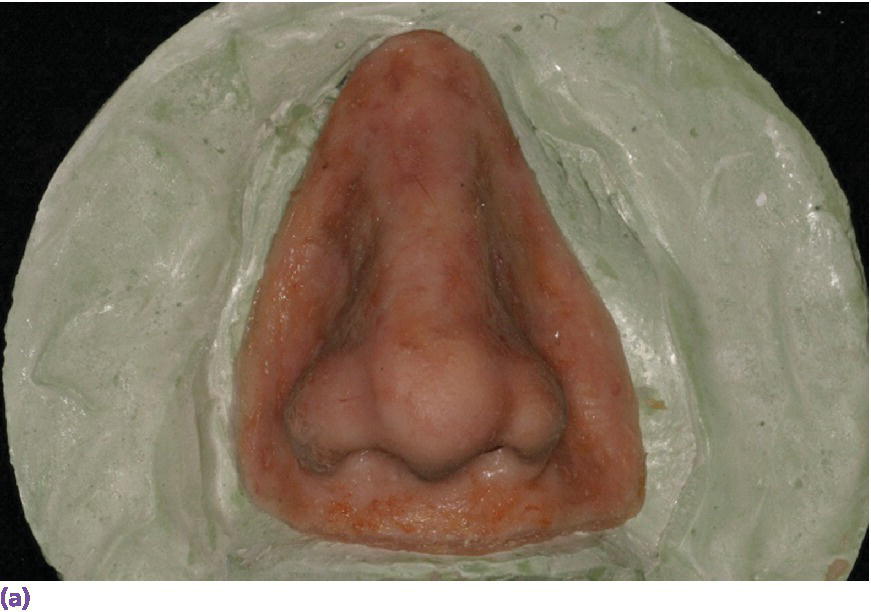
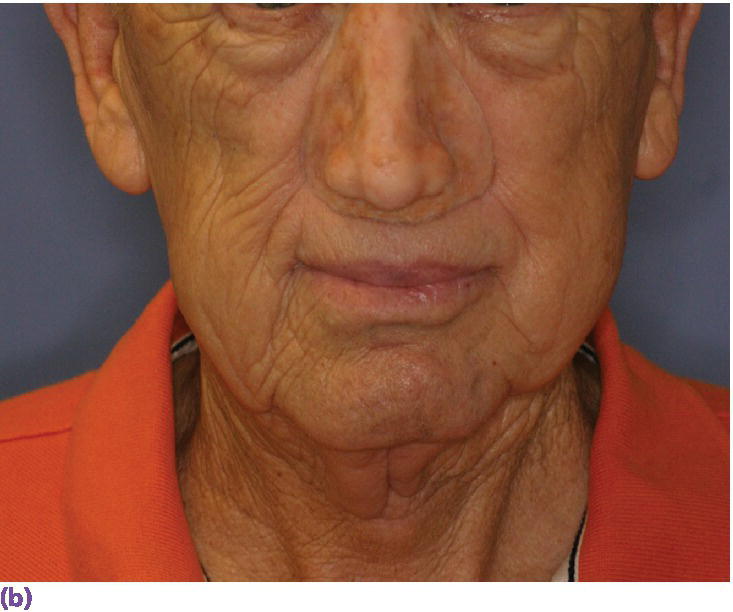
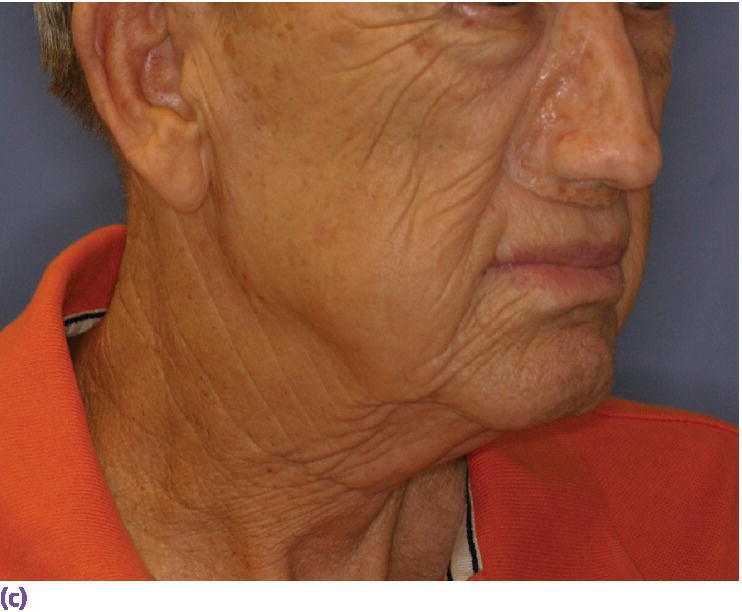
Figure 22.28 (a) Final nasal prosthesis on master cast. (b) Full face view of final nasal prosthesis positioned on patient’s face. (c) Three‐quarter view of final nasal prosthesis positioned on patient’s face.
Implant‐retained auricular prostheses
Patients with developmental and acquired defects of the auricle can elect to have no treatment, autogenous surgical reconstruction, or prosthetic reconstruction. Since 1986, carefully selected patients in North America have had the treatment option of the placement of craniofacial implants in the mastoid process in order to retain a prosthesis by the use of magnets or clips.
The presurgical planning must include appropriate CT radiographic imaging of the mastoid process. In addition, the presurgical planning includes a diagnostic waxing of the auricle that is converted to clear methyl methacrylate resin to be utilized as a surgical template. The templates are prepared in a manner to allow skin markings through holes where the implants will ideally be placed. A wider area can also be marked for alternative implant placement should a mastoid air cell be encountered at the time of surgery. The surgical procedure includes a full‐thickness skin flap and marking of the planned implant sites with methylene blue using needle injection. Tissue remnants, skin tags, or scar tissue should be removed. Care should be taken to thin the skin and subcutaneous tissue to 2 mm or less. When bone quality is very good, a one‐stage procedure can be utilized. The normal healing time for the mastoid process is 3–4 months when the bone quality is good and in the absence of irradiation of the bone. The healing time for irradiated patients or patients with poor bone quality is a minimum of 6 months.
A 36‐year‐old patient with congenital atresia of the right auricle had numerous unsuccessful plastic and surgical reconstructive attempts (Figure 22.29). The patient was referred to the University of North Carolina and elected to have an implant‐retained auricular prosthesis. A surgical template was utilized for guided surgical placement of the two craniofacial implants (Figure 22.30). The bar consisted of two magnets and a clip for the retention of the auricular prosthesis (Figure 22.31). The final silicone prosthesis was cast and custom stained (Figure 22.32). The prosthesis contained a superstructure with magnet keepers and a single clip for retention (Factor II Inc. Lakeside, AZ).
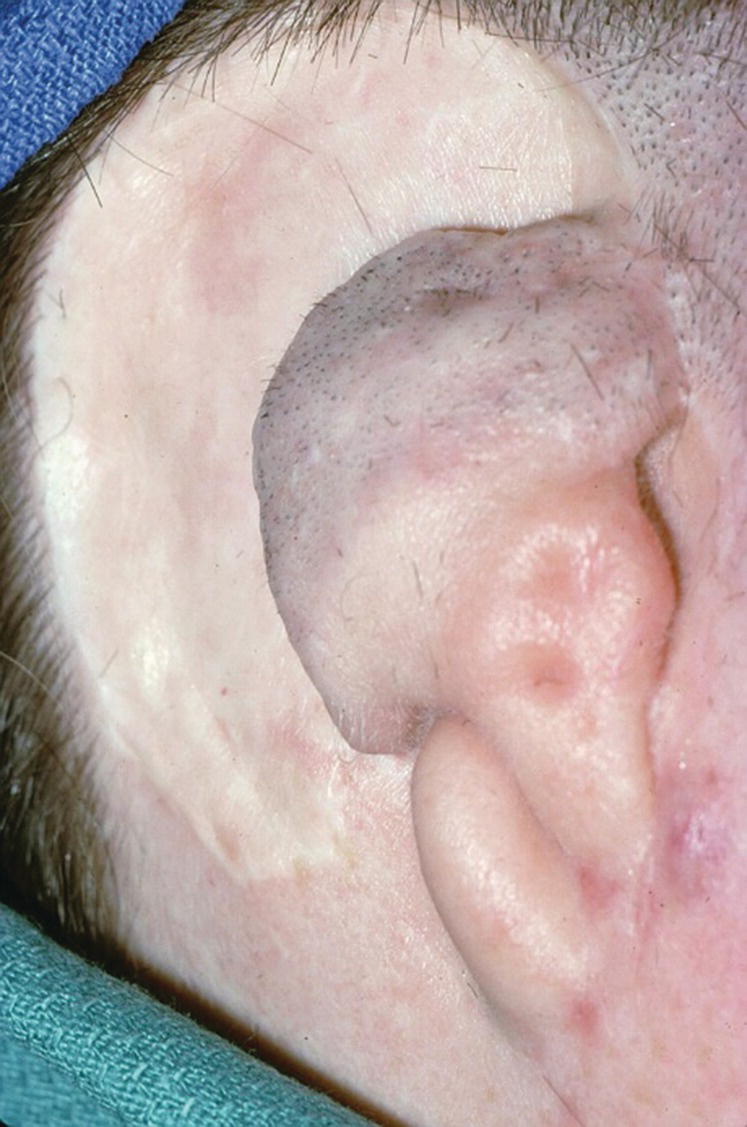
Figure 22.29 Patient with a history of congenital atresia and numerous plastic surgical reconstructions. Presurgical planning includes removal of tissue tags and thinning of the skin and subcutaneous tissue to 2 mm or less.
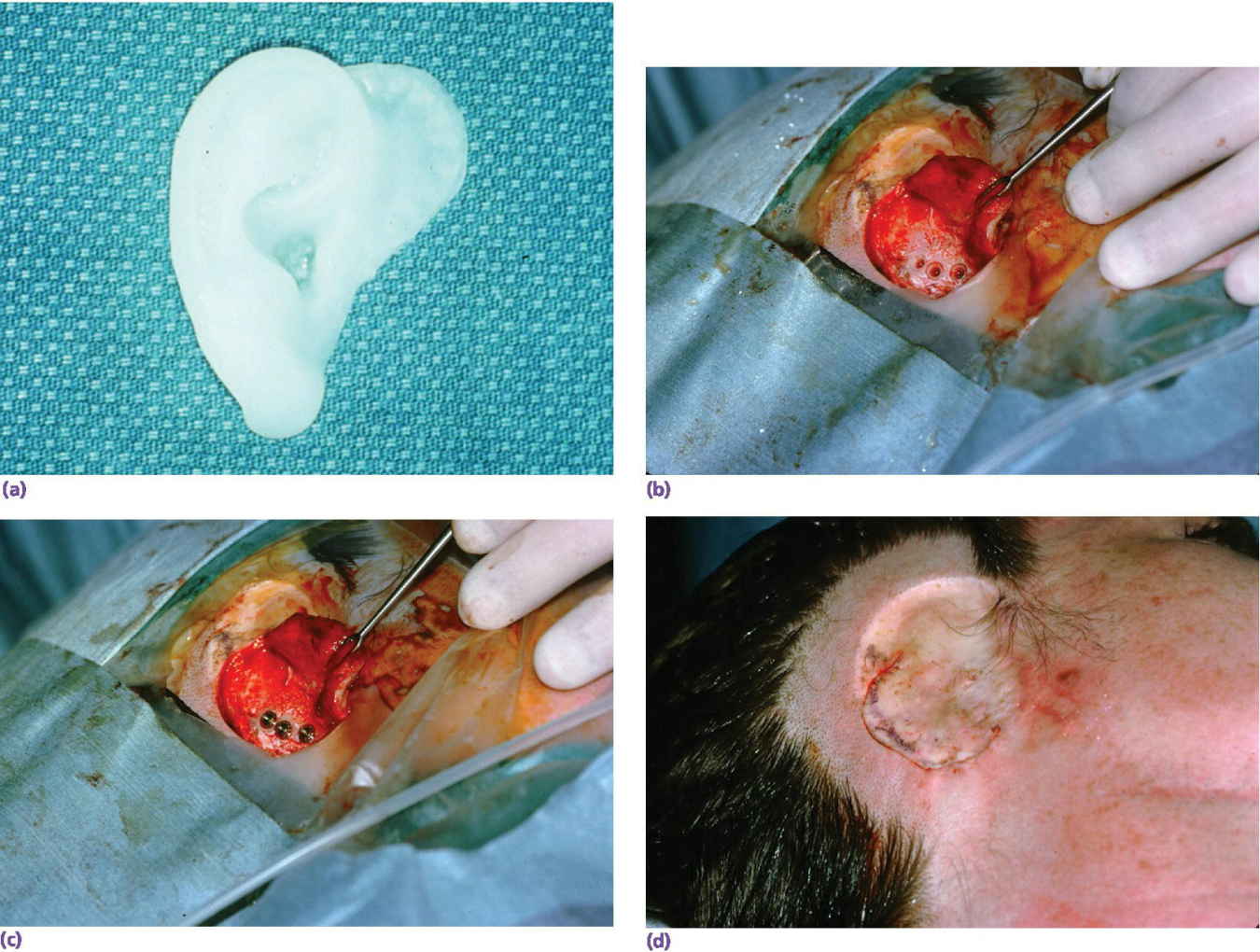
Figure 22.30 (a) Surgical template. (b) Three implants placed with countersinking. (c) Abutments connected to implants. (d) Surgical closure.
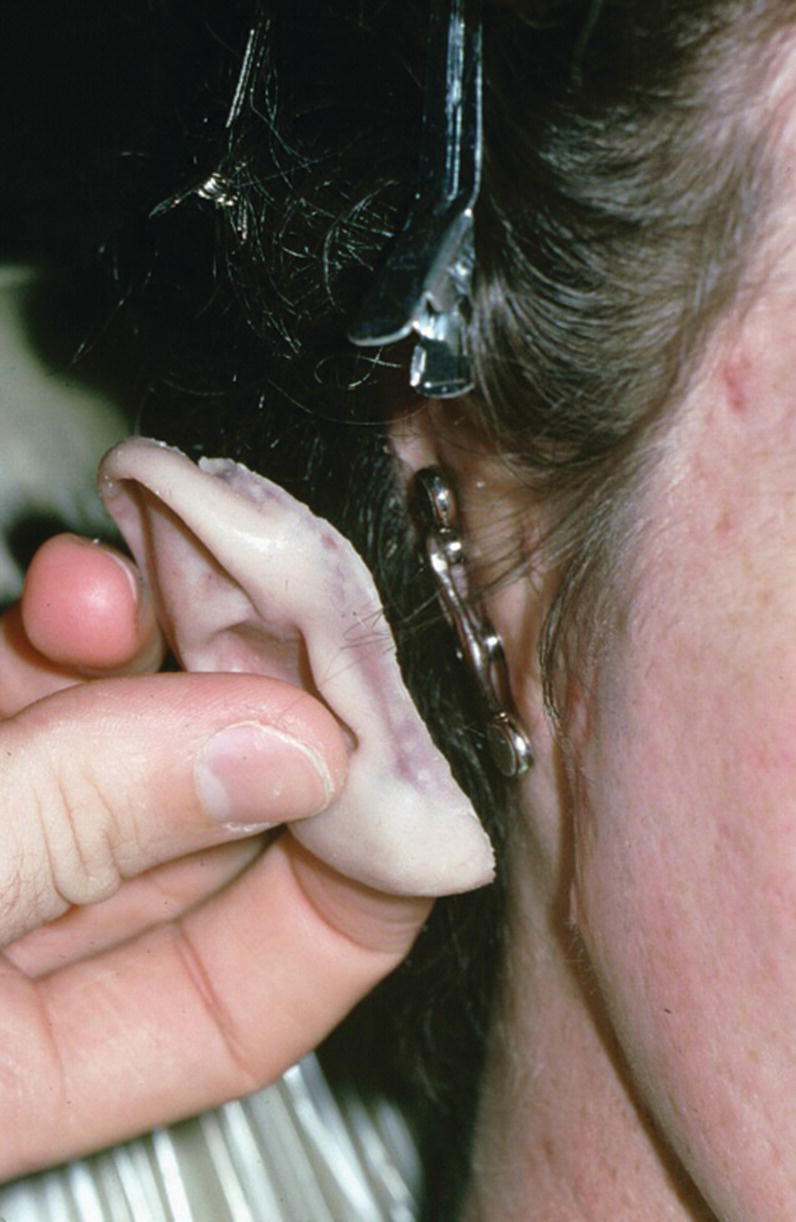
Figure 22.31 Bar framework consists of three magnets and two bar segments with keepers in the superstructure.
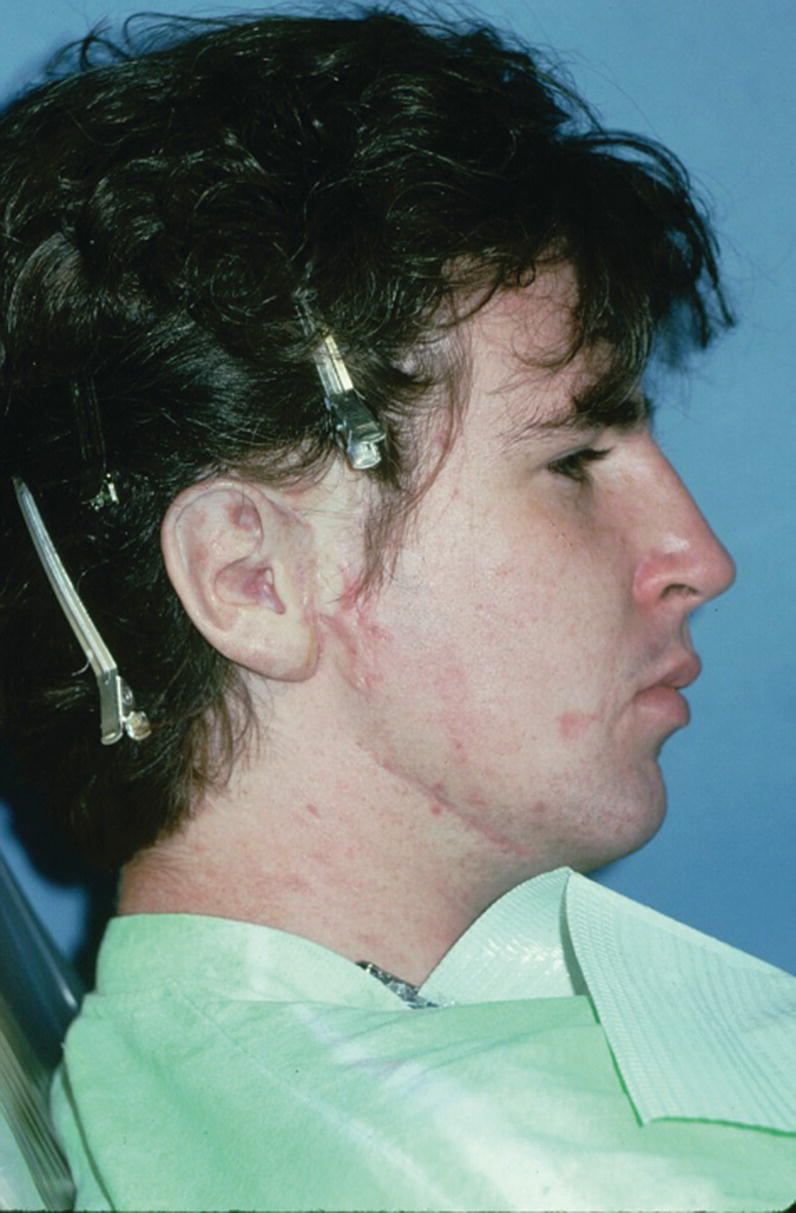
Figure 22.32 Final auricular prosthesis cast, custom stained, and engaged onto implant framework.
A 76‐year‐old patient lost his left auricle from recurrent basal cell carcinoma. The surgical procedure included excision of the left auricle, left parotidectomy, and partial reconstruction using a deltopectoral flap. The patient was treated postoperatively with radiation therapy. In anticipation of the planned craniofacial implants, a radiation shield was fabricated to protect the implant sites and allow delivery of the radiation to the tumor site.44 Three implants were placed 4 months after radiation utilizing a surgical template (Figure 22.33). Three months’ healing time was allowed before loading. The Magnacap magnet system was utilized (Technovent Ltd, Leeds, UK). Magnet keepers were oriented and connected using autopolymerizing acrylic resin (Figures 22.34 and 22.35). The prosthesis was intrinsically colored and processed in silicone (A2186; Factor II Inc., Lakeside, AZ) (Figure 22.36).44
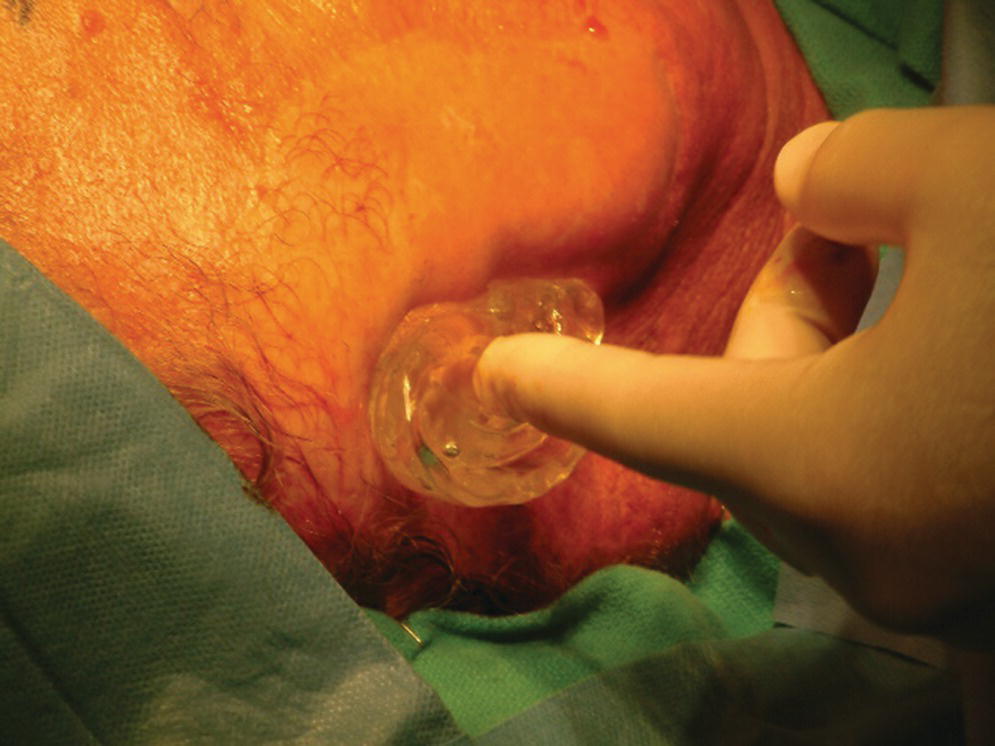
Figure 22.33 Surgical guide oriented on face.
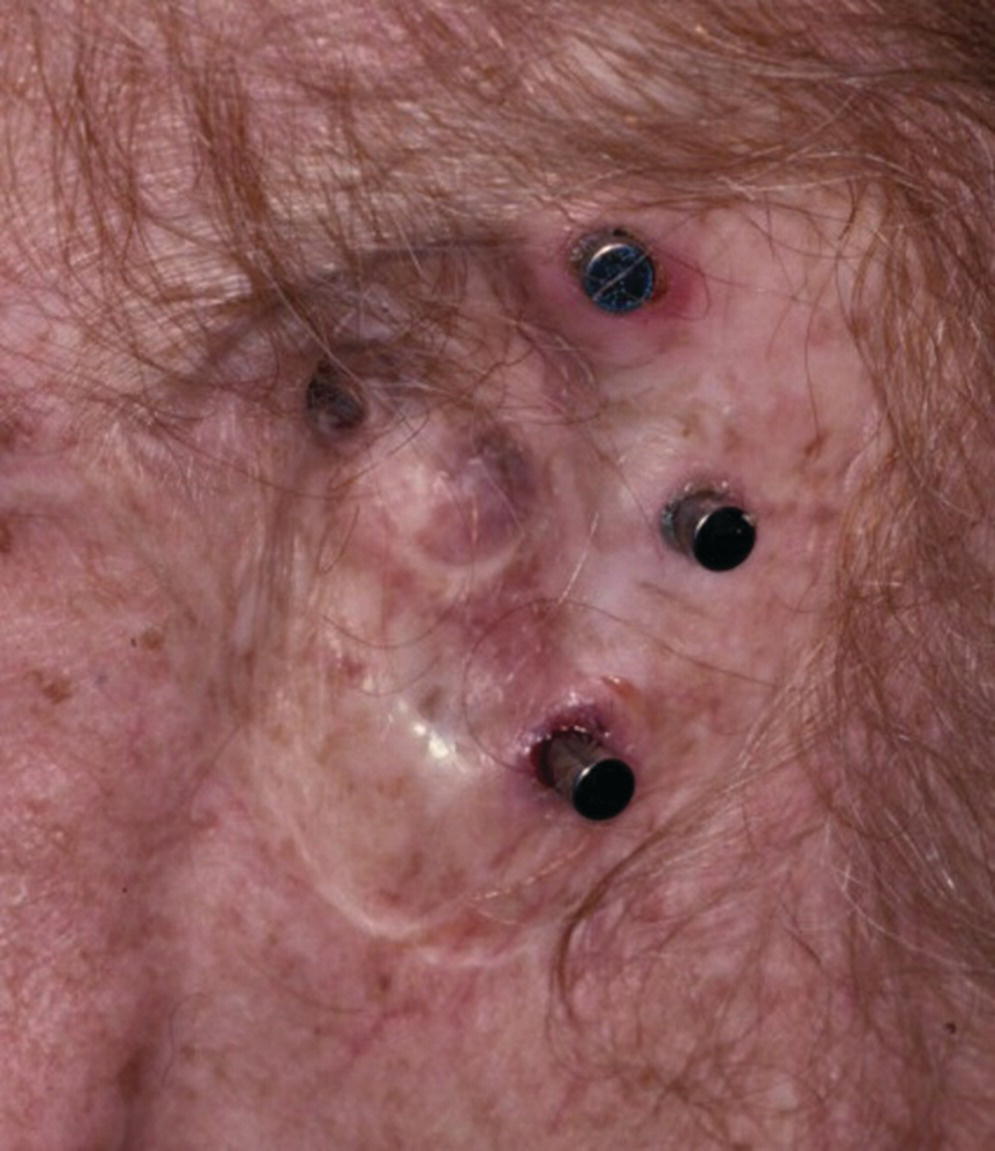
Figure 22.34 Implant healing abutments in place after 3 months of healing.
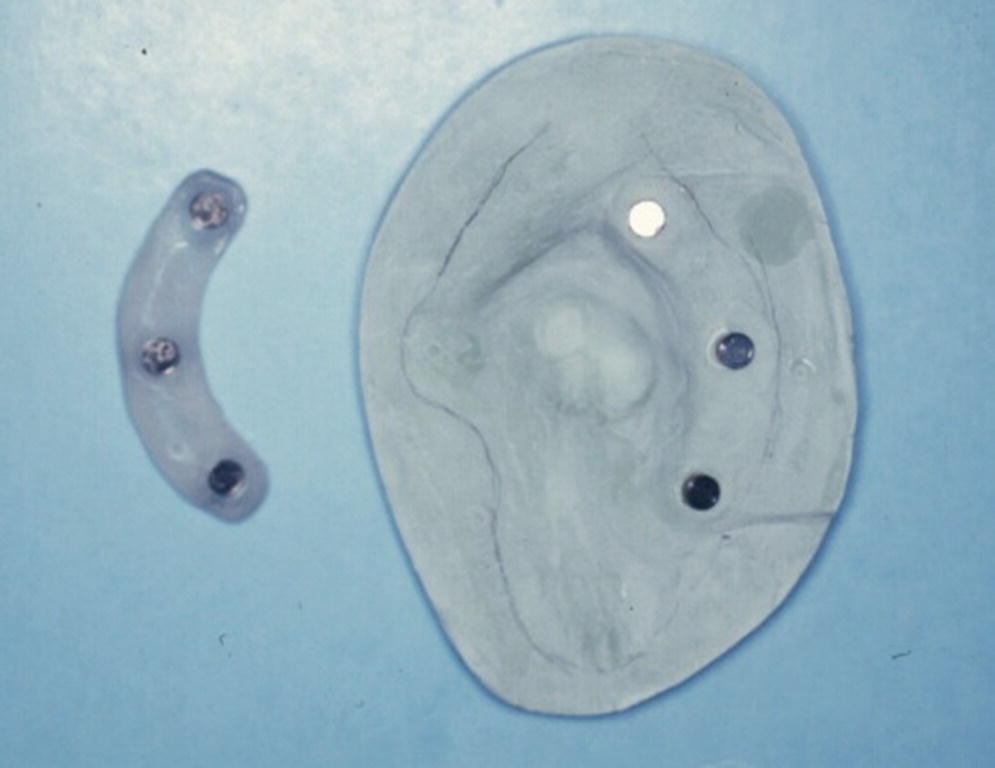
Figure 22.35 Magnet keepers oriented with autopolymerizing acrylic resin on master cast.
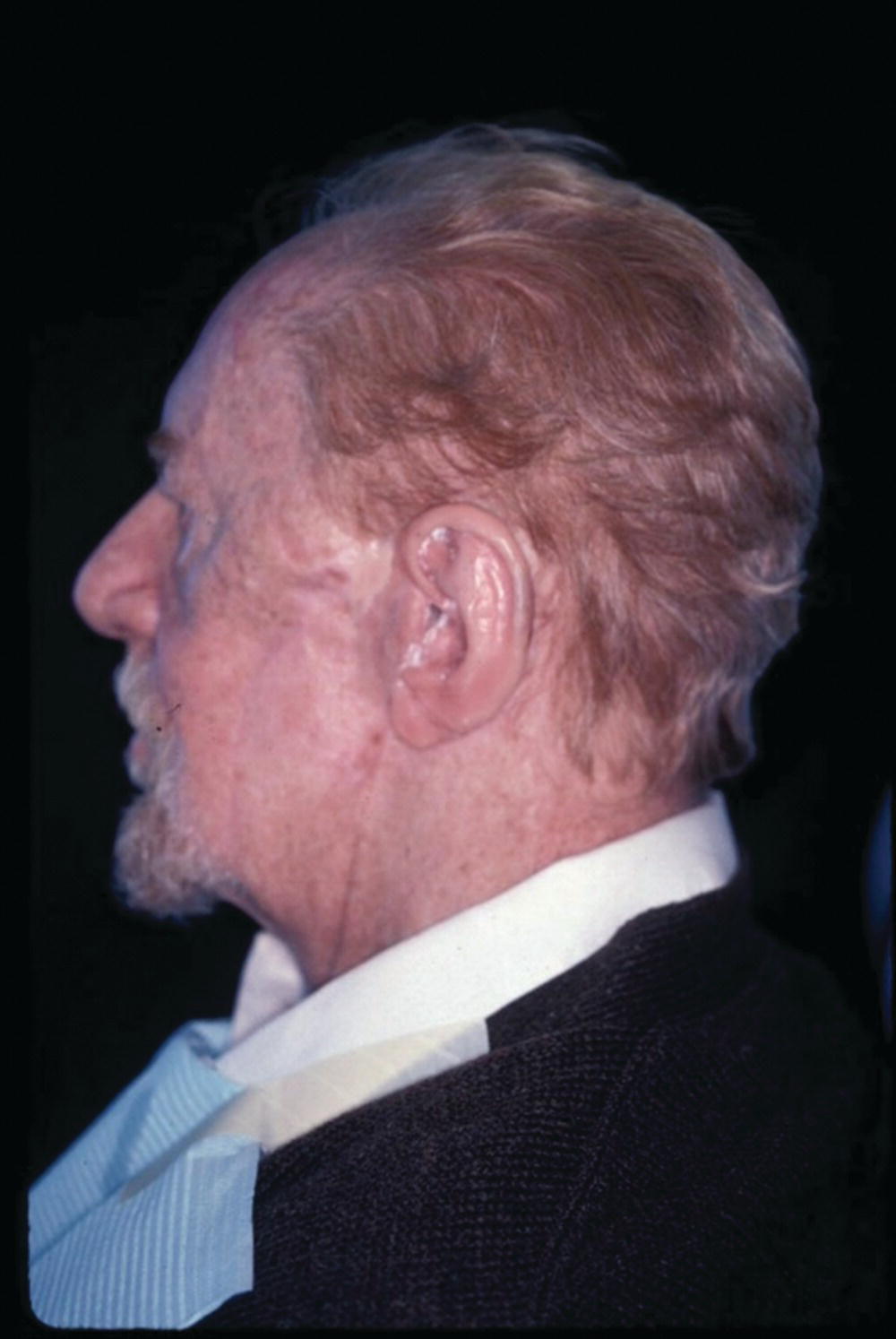
Figure 22.36 The auricular magnet‐retained prosthesis after custom staining.
Source: Hatfielt et al., 2001.44 Reproduced with permission from Elsevier.
Implant‐retained orbital prostheses
Surgical reconstruction for orbital resections is a challenge. Soft tissue flaps to close the orbital contents are not esthetic. Therefore the use of craniofacial implants to retain an orbital prosthesis is a good treatment option if patients are aware that the survival rate of orbital implants is less than that for implants in the mastoid process used to retain auricular prostheses.15
The presurgical planning must include a prosthodontist as part of the team since the implants must be carefully planned to be within the confines of the prosthesis and the retentive components and implants must not limit the esthetics of the final facial prosthesis. The presurgical planning should also include a CT radiographic image of the orbit, a diagnostic waxing, and a surgical template (Figure 22.37). The frontal sinus can be the limiting factor for placement of implants in the 1 and 2 o’clock positions for the right orbit and at the 10 and 11 o’clock positions for the left orbit. The supraorbital rim is the most common site for orbital implant placement.
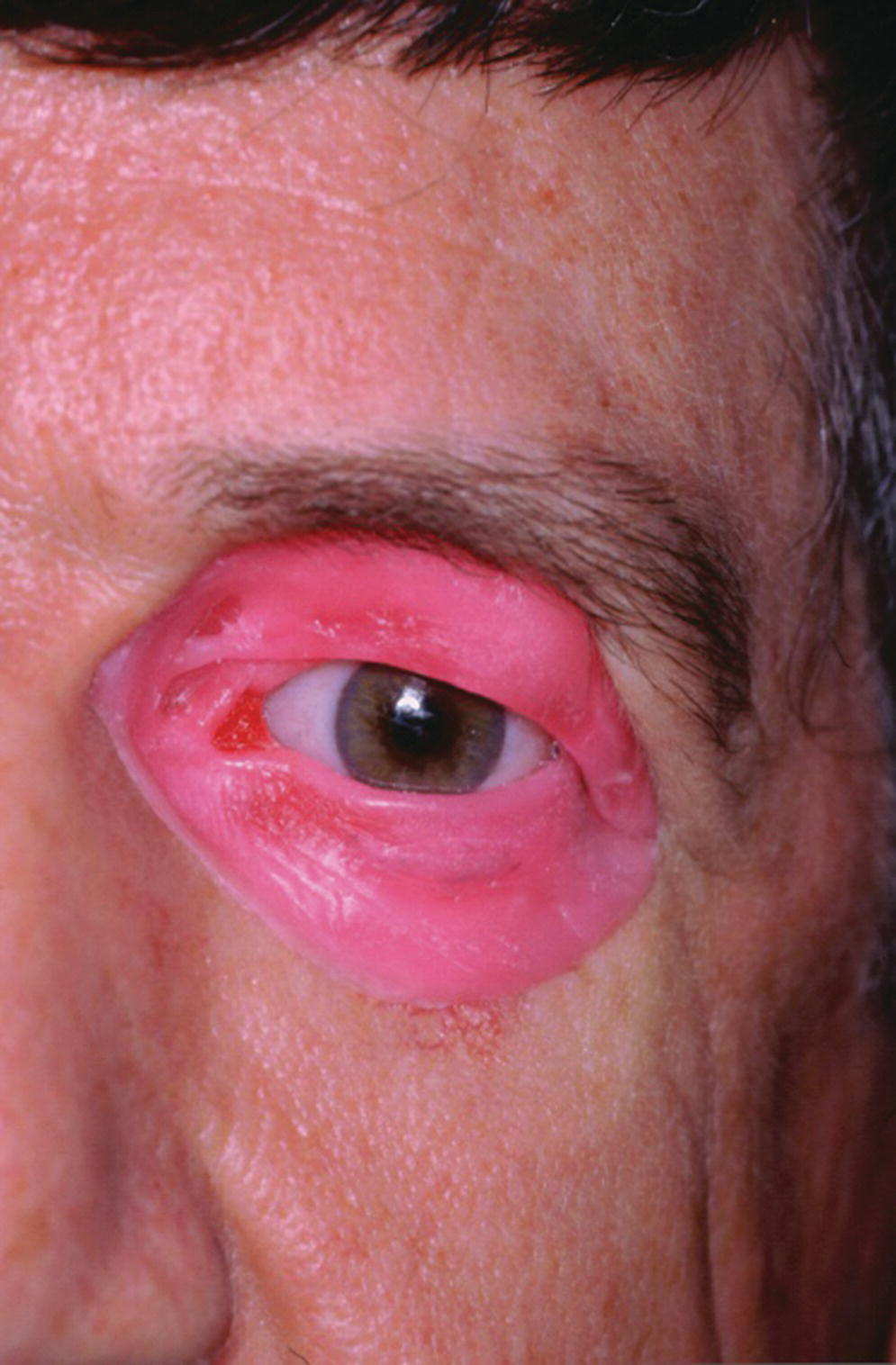
Figure 22.37 Diagnostic waxing for presurgical planning.
Surgical exoneration due to cancer is the most common cause of the loss of the orbital contents (Figure 22.38). In the patient in Figure 22.38, three craniofacial implants were placed in the supra‐ and infraorbital rims and a bar was cast to hold three magnets for retention. The orbital prosthesis has a clear acrylic magnet keeper (substructure) embedded in the intaglio surface to engage the three magnets on the framework for retention (Figure 22.39). The prosthesis provides the patient with a very esthetic restoration of the orbit (Figure 22.40). The custom ocular prosthesis is contained within the orbital prosthesis.
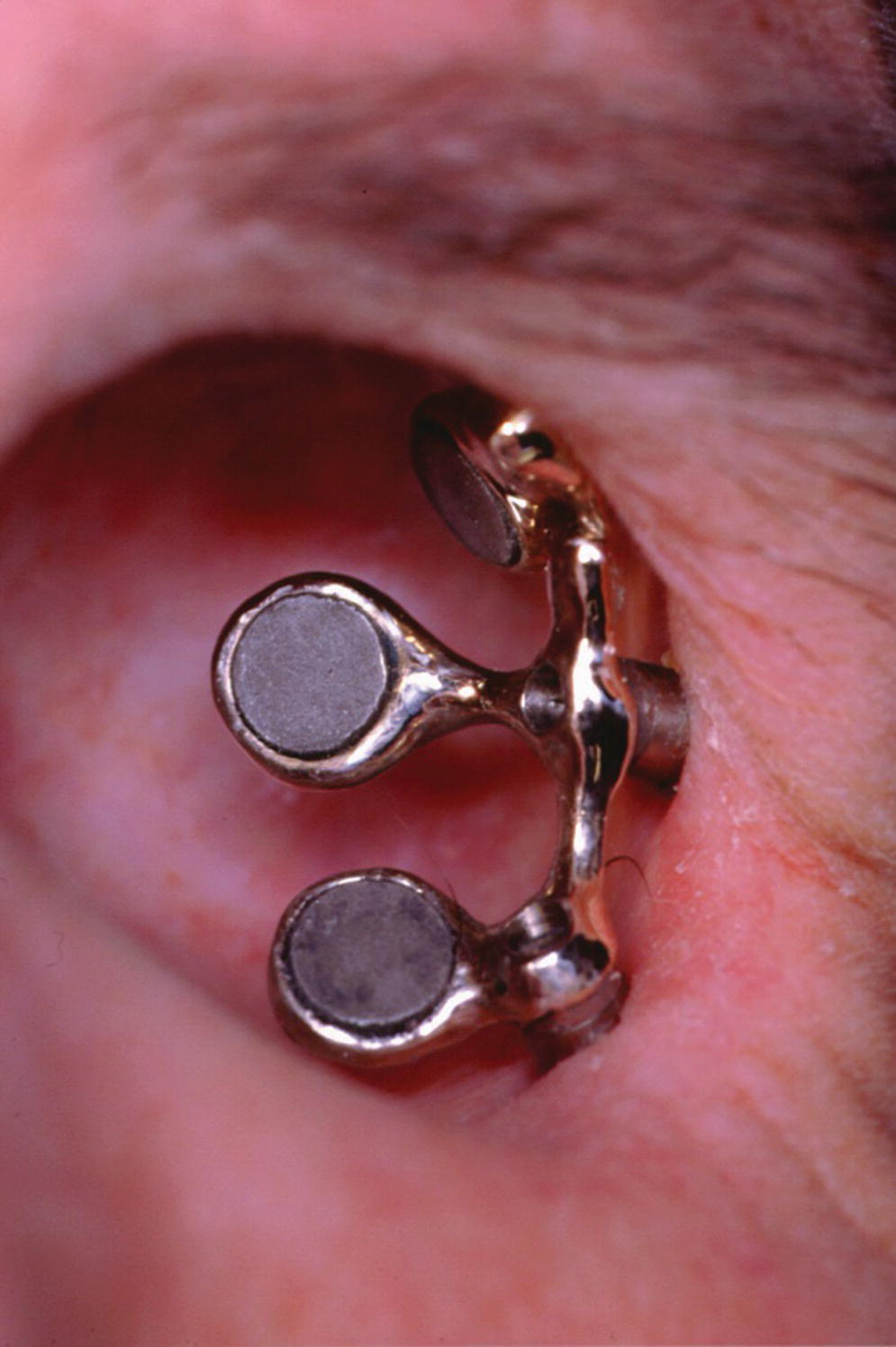
Figure 22.38 Three implants placed in the supraorbital rim and infraorbital rim with a cast bar containing three magnets for retention.
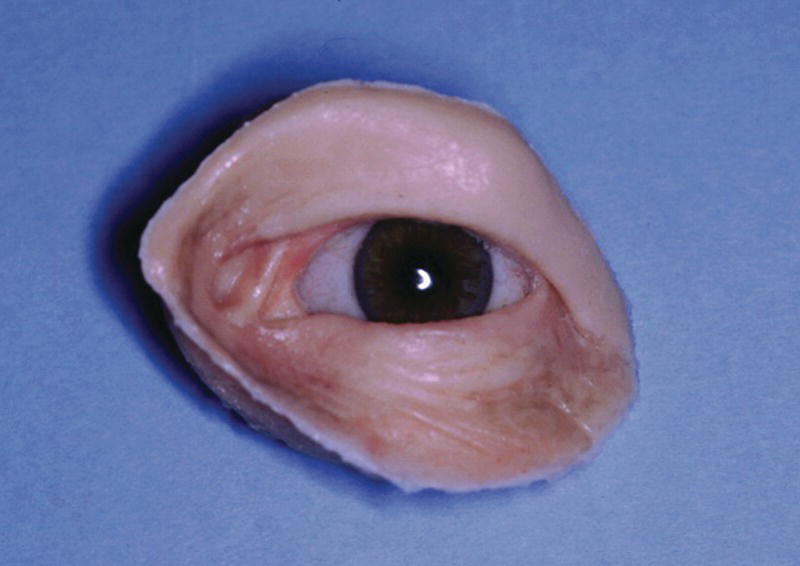
Figure 22.39 The custom orbital prosthesis which contains a custom ocular prosthesis and the substructure with the magnet keepers.

Figure 22.40 The orbital prosthesis is retained by magnets.
A large orbital resection was necessary due to a malignancy (Figure 22.41). The patient was treated with the placement of standard intraoral implants (IMZ). A bar was cast in two sections with a nonrigid connector due to the divergent paths of insertion (Figure 22.42). The bar was cast to hold three magnets for retention (Figure 22.43). A custom ocular prosthesis was fabricated to match the patients left eye. The ocular prosthesis was positioned using this calibrated measuring device (Figure 22.44). The magnet keeper (infrastructure) was housed in the intaglio surface of the orbital prosthesis (Figure 22.45). The wax trial of the orbital prosthesis was verified on the patient (Figure 22.46). The intaglio surface of the orbital prosthesis contained the infrastructure with the magnets (Figure 22.47). The final prosthesis restored the patient’s orbital defect and improved this patient’s quality of life (Figure 22.48). The margins of the final prosthesis are disguised by the patient’s selection of eyeglasses (Figure 22.49).
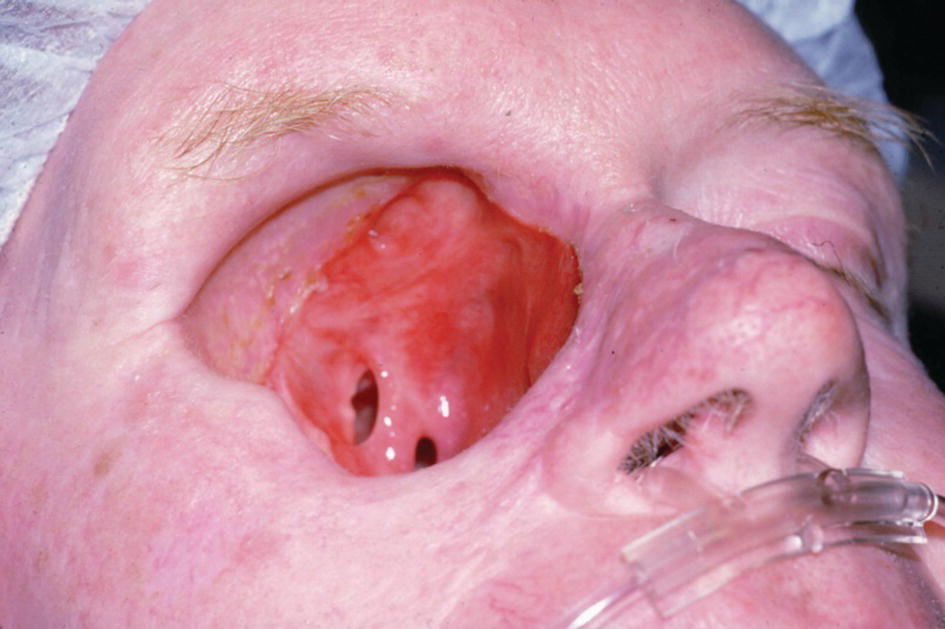
Figure 22.41 Large orbital defect due to a malignancy.
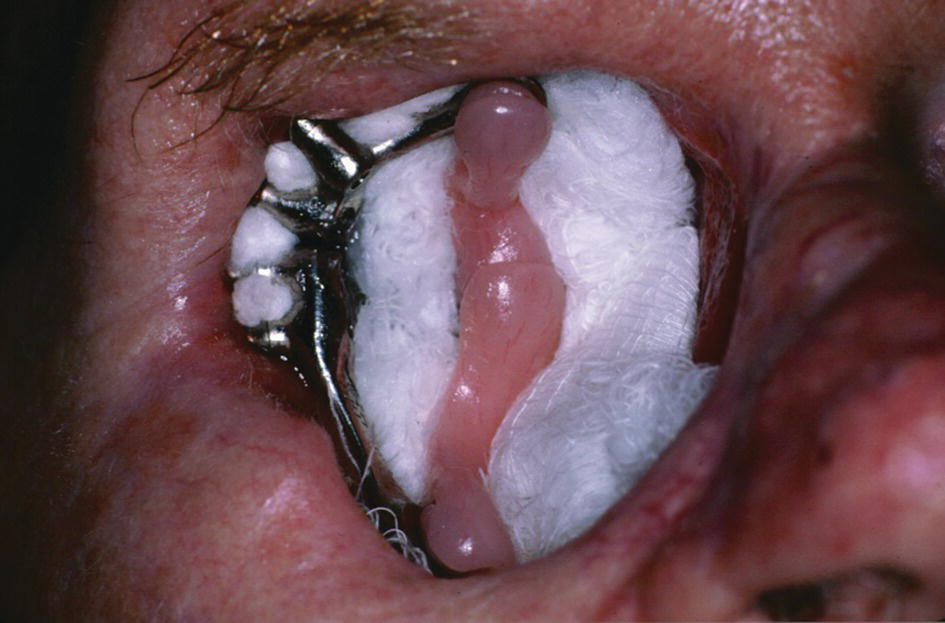
Figure 22.42 Four IMZ implants are placed to anchor a bar with magnets
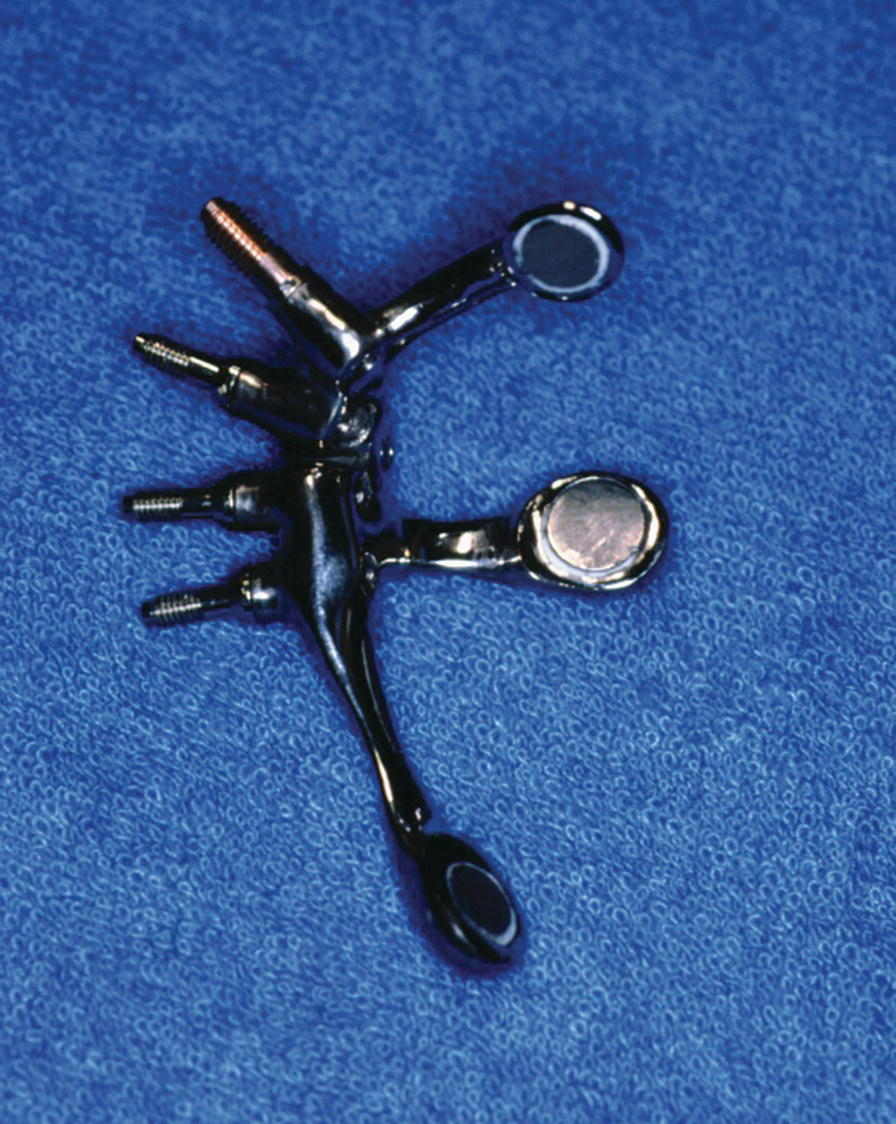
Figure 22.43 The bar has three magnets to aid in retention
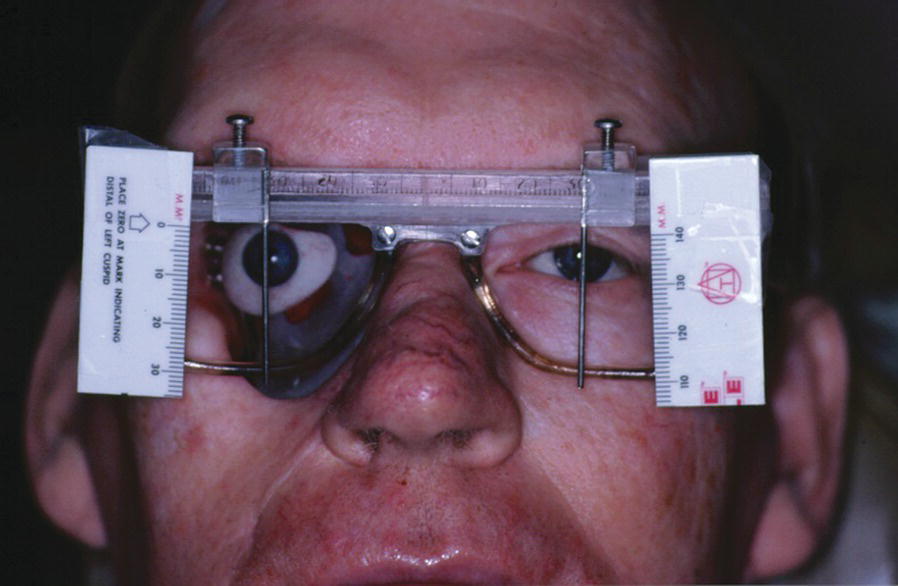
Figure 22.44 Positioning of the ocular prosthesis with a calibrated device.
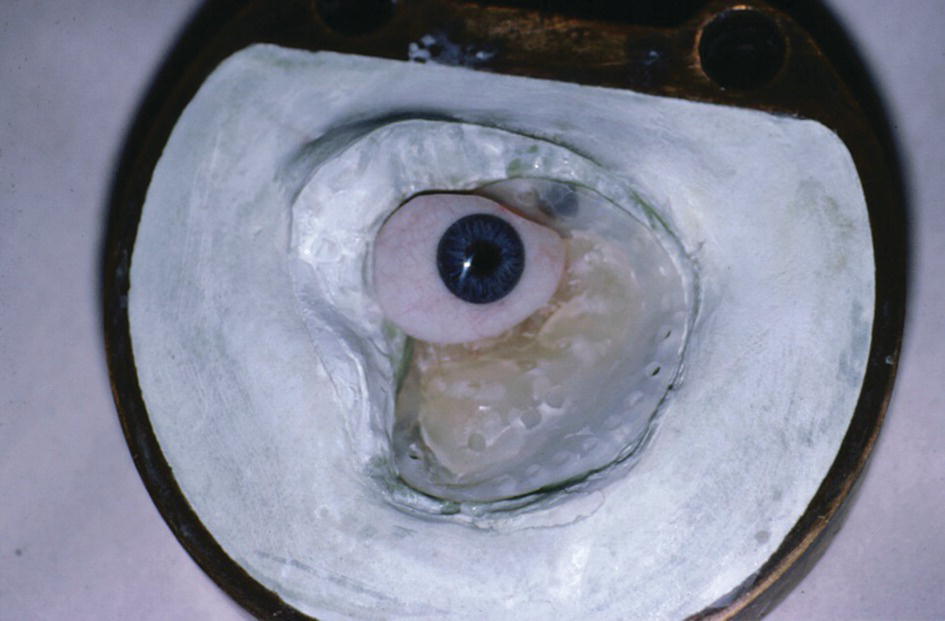
Figure 22.45 The infra‐structure is the magnet keeper.
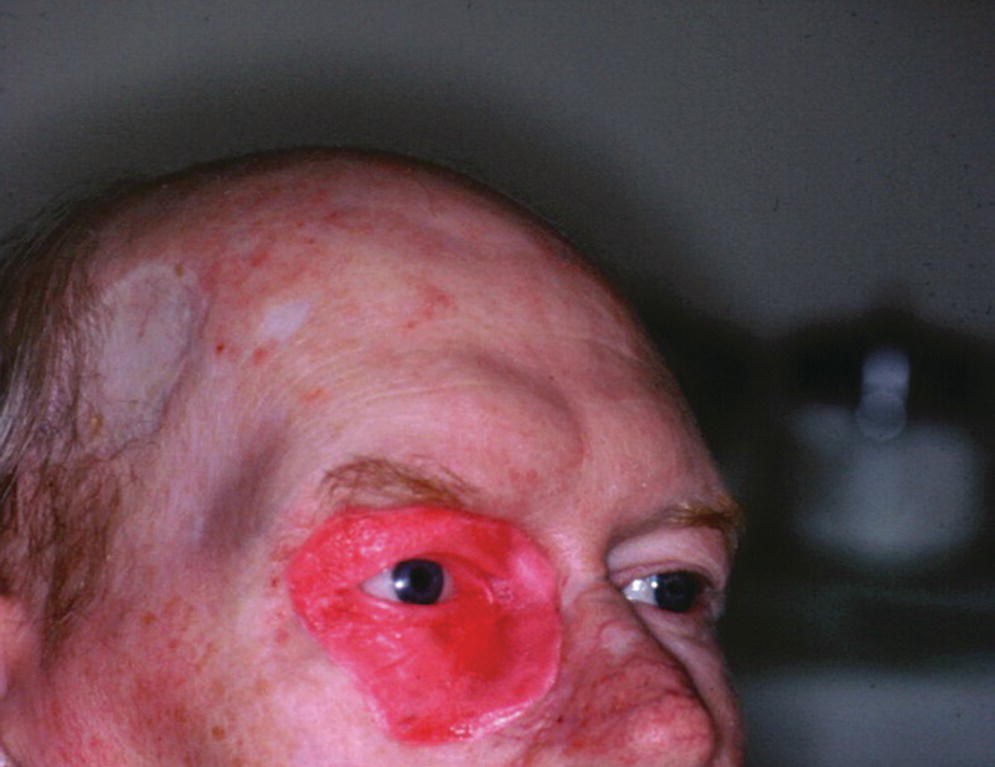
Figure 22.46 Wax trial of the orbital prosthesis.
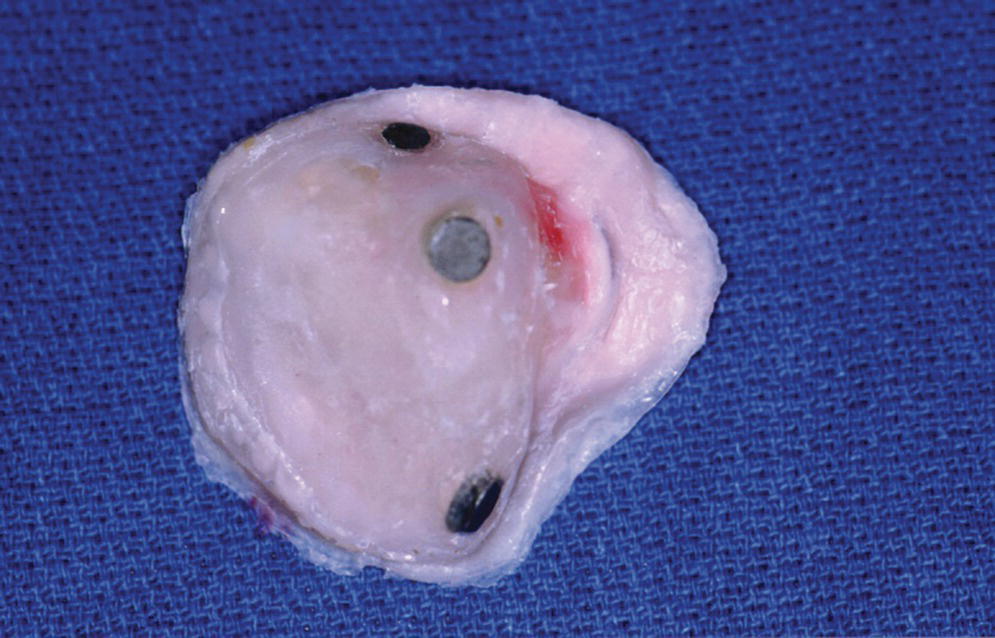
Figure 22.47 The intaglio surface of the orbital prosthesis with the magnet keeper as the substructure.

Figure 22.48 The orbital prosthesis is retained by magnets.

Figure 22.49 Eye glasses disguise the margins of the prosthesis.
Midfacial prostheses
Midfacial defects are facial defects of the middle third of the face that have communication with the oral cavity. Midfacial defects are defined as either lateral or midline. A lateral defect would extend to the cheek and have communication with the oral cavity. An example of a midline midfacial defect would be a combination nasal defect and/or upper lip defect and communication with an intraoral maxillary defect. The types of maxillofacial defects can be quite challenging due to facial defects bordering on movable tissue that limits esthetics, retention, and support.
These midfacial defects are complicated, since any intraoral prosthesis movement will be transmitted to the extraoral prosthesis (Figure 22.50). In 1975, the patient was diagnosed with advanced squamous cell carcinoma at the age of 42. The ablative surgery included a total rhinectomy, bilateral maxillectomies, and bilateral neck dissections. His midfacial defect extended to his left and right cheeks. His ablative surgery was followed by a full course of external‐beam radiation. The patient wore a poorly retained maxillary obturator and facial prosthesis held on with two straps fastened around his head.
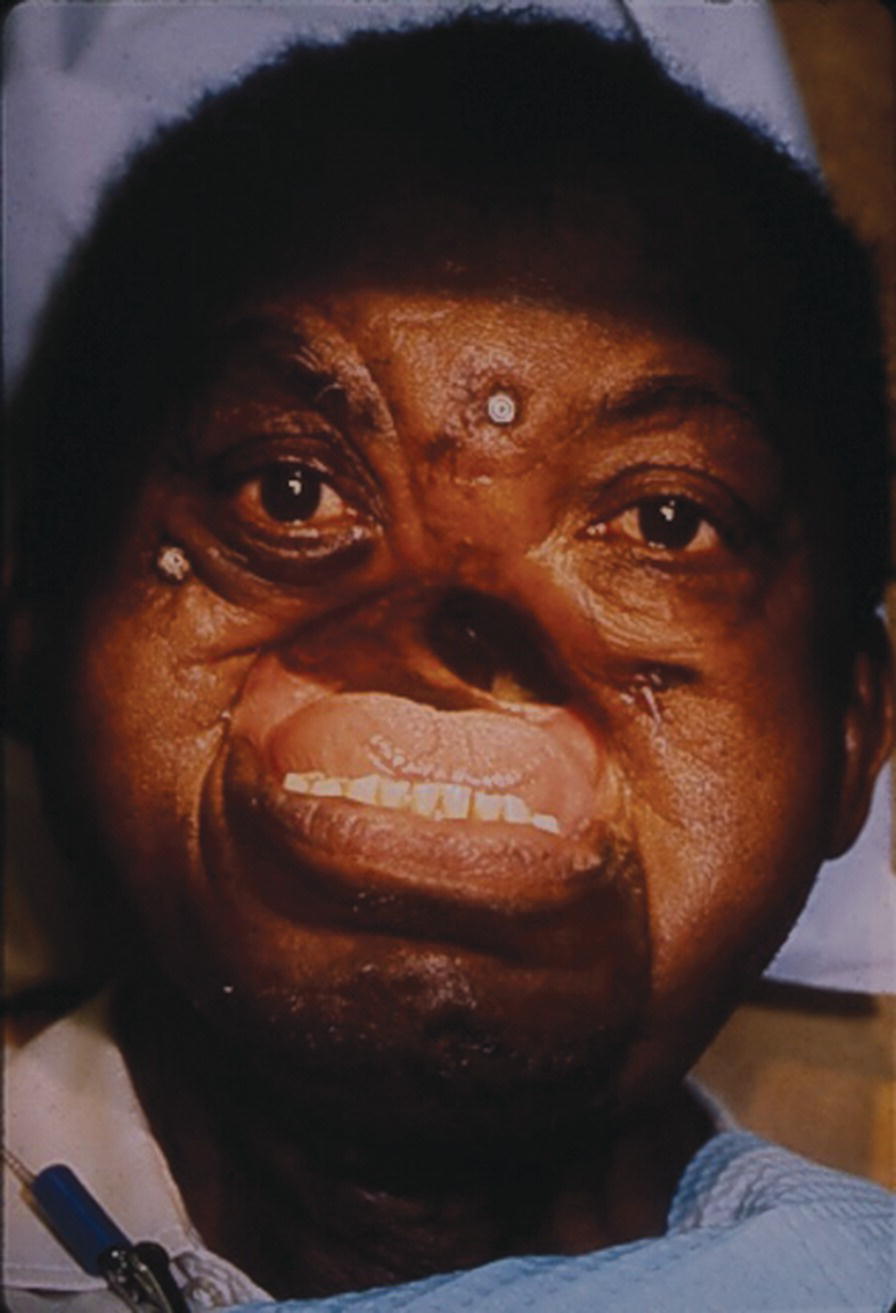
Figure 22.50 Two implants placed in the zygoma and one in the frontal bone.
Source: Evans et al., 1996.45 Reproduced with permission from Elsevier.
Presurgical implant planning was coordinated with the Department of Oral and Maxillofacial Surgery at Bronx V.A. Medical Center affiliated with Columbia University College of Dental Medicine. The presurgical planning included radiographic imaging of the craniofacial area. The patient was 63 years old when he received two 7 mm Branemark implants (Nobelpharma USA, Chicago) into the frontal bone and right zygoma and a 10 mm Branemark implant into the left zygoma. The implants were placed in two stages and 6 months’ healing time was allowed since the patient had received radiotherapy. At stage two, 8.5 mm standard abutments were added to the implants. Three weeks’ healing time was allowed after stage 2 uncovering before square impression abutments were attached and picked‐up utilizing a custom tray and two‐stage impression technique. The framework was cast in palladium–gold alloy (Legacy, J.F. Jelenko and Co., Armonk, NY) (Figure 22.51). The obturator was fabricated utilizing standard impression techniques and the vertical maxillomandibular relationships were established by the use of esthetics phonetics, and swallowing. A trial arrangement was performed to verify the esthetics and centric relation. The palladium–gold framework had four regions designed to engage retentive Dolder clips on the obturator (Figure 22.52). The silicone prosthesis was retained by a castable O‐ring attachment system (Implant Innovations Inc., West Palm Beach, FL). The pattern was waxed and cast onto the gold alloy framework. The three patrix attachments were at the terminal ends of the framework. The matrix O‐ring attachments were oriented to the intaglio surface of the facial prosthesis by mesh and acrylic resin. Three magnets (Shiner SR, Preat Corp., San Mateo, CA) were attached to the nasal region of the obturator and oriented to three magnets on the intaglio surface of the facial prosthesis (Figure 22.53). The patient was able to place and remove the facial prosthesis. The use of craniofacial implants improved this patient’s quality of life and it should be noted that he sang in his church choir.45
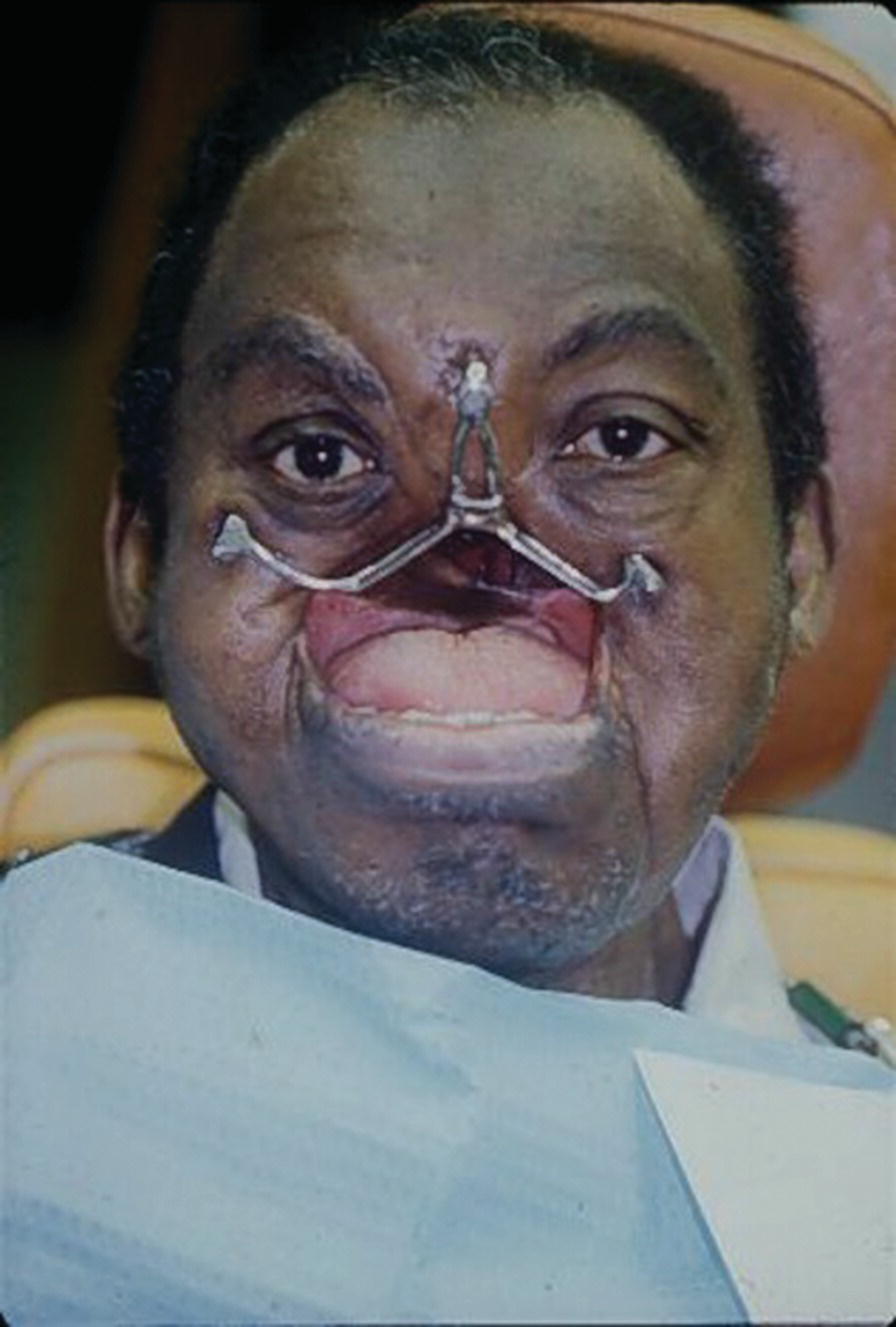
Figure 22.51 The framework is cast in a gold–palladium allow. There are three O‐ring attachments.
Source: Evans et al., 1996.45 Reproduced with permission from Elsevier.
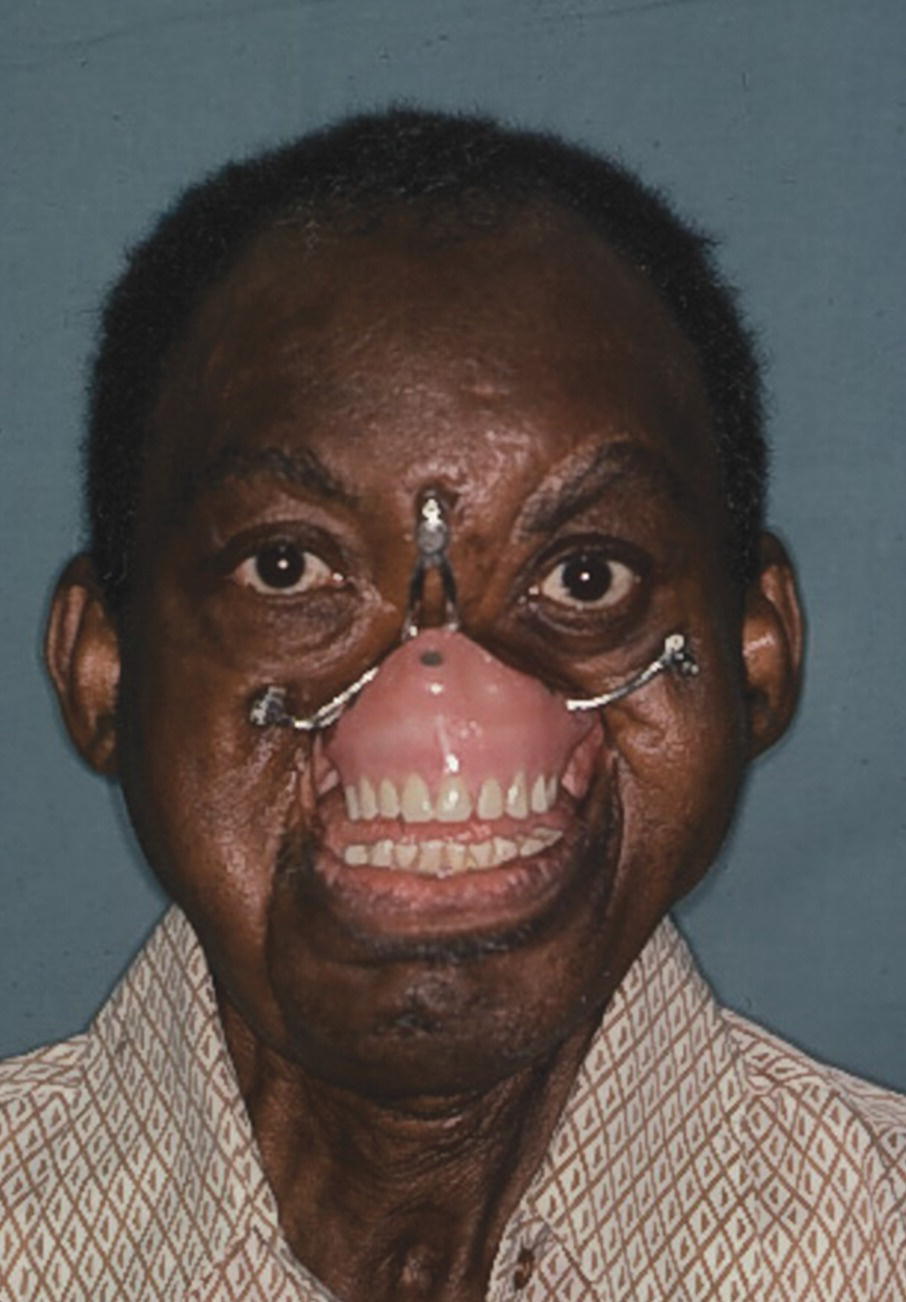
Figure 22.52 The obturator is retained by four Dolder clips.
Source: Evans et al., 1996.45 Reproduced with permission from Elsevier.

Figure 22.53 The facial prosthesis snaps onto the O‐rings and engages the three magnets in the obturator in the nasal area.
Source: Evans et al., 1996.45 Reproduced with permission from Elsevier.
Related advances in technology for cranionfacial implant‐retained restorations
Computer‐guided implant placement had its beginnings in intraoral prosthodontics. However, the same concept has been demonstrated in extraoral prosthetics. Van der Meer et al.46 described digital presurgical planning of the implant placement and the achievement of a satisfactory surgical and prosthodontic result. The use of rapid prototyping (RP), stereolithography (STL), and modeling have allowed for digital design of maxillofacial prostheses. From imaging techniques like CT and magnetic resonance imaging (MRI), hard and soft tissues can be captured and the information converted using software into a RP or STL model. When indicated, mirror imaging of the unaffected side can be utilized to assist in the reconstruction of the craniofacial defect. Digital files of noses and other facial structures could be helpful in creating prostheses when native tissues are lost due to trauma.
Traditional moulage techniques have the disadvantage of distortion and inaccuracy. CT and MRI for sterolithography have the disadvantage of not capturing soft tissue detail. Recent advances in the area of craniofacial prostheses have been reported by the US Naval Postgraduate Dental School in Bethesda where digital capture of the facial structures with photogrammetry techniques has been succesful. Image capture, using 3dMDfaceTM Imaging, is fast and captures tissues in their natural state. The 3‐D image capture has been utilized in the fabrication of a facial prosthesis by taking the photo data and storing it in a format to fabricate rapid prototype models. The files are exported as a virtual reality modeling language file (VRML). The VRML files are imported to create both a STL and 3‐D printed RP model. The RP models can be duplicated and the duplication poured in type 4 stone for prosthesis fabrication.47,48 Advances in digital technology are having a remarkable impact in the specialty of prosthodontics by providing patients with high‐quality implant‐retained prostheses.
Summary
There are numerous advantages of implant‐retained craniofacial prostheses over the conventional adhesive‐retained prostheses. The use of implants to restore facial defects caused by trauma, disease, or congenital abnormality has had a profound impact and revolutionized the subspecialty of maxillofacial prosthetics. A review of the classic literature provides the understanding of how craniofacial implant‐retained restorations developed and came to North America in the mid 1980s. The classic and current literature provides a discussion of challenges with implant‐retained prostheses, such as bone quality, irradiation versus no radiotherapy, dosage of radiation, survival rate due to site of implant placement, anatomic limitations, soft tissue complications, and prosthetic limitations due to improper implant placement. Presurgical planning for the craniofacial implant‐retained prosthesis is critical, just as appropriate presurgical planning is required for all dental implants. The surgical placement of craniofacial implants has been reviewed. Important prosthodontic principals for nasal, auricular, orbital, and midfacial prostheses has been provided. Emerging technology in the area of presurgical planning, digital capture of the facial defect and design of the definitive craniofacial prosthesis continues to change the traditional practice of maxillofacial prosthetics. The advances of implant‐retained craniofacial prostheses and emerging technology have had an impact on these patients’ quality of life.
Postscript
A few months after Dr. Robert Ferguson Wright, lead author, submitted this chapter, he died suddenly at his home in Chapel Hill, North Carolina. He was a valued friend as well as a gifted educator and clinician. His penchant was a devotion to life‐long learning while generously contributing to the scientific community. After completing his DDS degree at the University of Memphis, he continued his postgraduate education in prosthodontics at LSU Health Sciences Center and a maxillofacial prosthetics residency at Memorial Sloan Kettering Cancer Center. He held academic positions at Columbia University, Harvard University, and, most recently, at University of North Carolina. Robert was an active participant in the American College of Prosthodontics, the Academy of Prosthodontics, the Greater New York Academy of Prosthodontics, and the American Society of Dental Educators. He possessed an exceptional balance between his head and his heart and was a role model for his students and colleagues. I am most grateful that his scholarly work is featured in this chapter serving as a testament to his craft.
Steven J. Sadowsky
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


