Chapter 21
Dental Amalgam
21.1 Introduction
An amalgam consists of a mixture of two or more metals, one of which is mercury. Dental amalgam consists, essentially, of mercury combined with a powdered silver–tin alloy. Mercury is a liquid at room temperature and is able to form a ‘workable’ mass when mixed with the alloy. This behaviour renders the material suitable for use in dentistry.
The reaction between mercury and alloy which follows mixing is termed an amalgamation reaction. It results in the formation of a hard restorative material of silvery-grey appearance. The colour generally limits its use to those cavities where appearance is not of primary concern (see Fig. 21.1).
Dental amalgam has been used for many years with a large measure of success. For many years it was the most widely used of all filling materials. For various reasons, including the development of viable alternatives based upon resins and ceramics and perceptions of a dubious and frequently questioned level of safety, its popularity has declined.
21.2 Composition
Mercury used in dental amalgam is purified by distillation. This ensures the elimination of impurities which would adversely affect the setting characteristics and physical properties of the set amalgam.
The composition of the alloy powder is controlled by the ISO Standard for dental amalgam alloy (ISO 1559). The compositional limits allowed by the standard are given in Table 21.1. It can be seen that the major components of the alloy are silver, tin and copper. Small quantities of zinc, mercury and other metals such as indium or palladium may be present in some alloys. The compositional limit specified in the earlier version of the ISO Standard represented an attempt to control properties such as corrosion and setting expansion in the absence of any real understanding of the structure of amalgam. Materials having a composition which is in line with the pre-1986 standard are referred to as ‘conventional’ amalgam alloys. The change in the compositional limits specified in the current standard (post-1986) reflects a marked improvement in the understanding of structure–property relationships for the materials.
The quantities of silver and tin specified ensure a preponderance of the silver/tin intermetallic compound Ag3Sn. This compound, known as the γ (gamma) phase of the silver–tin system, is formed over only a small composition range and is particularly advantageous since it readily undergoes an amalgamation reaction with mercury. Most conventional alloys contain around 5% copper, which has a significant strengthening effect on the set amalgam.
The role of zinc is as a scavenger during the production of the alloy. The alloy is formed by melting all the constituent metals together. At the elevated temperatures required for this purpose there is a tendency for oxidation to occur. Oxidation of tin, copper or silver would seriously affect the properties of the alloy and amalgam. Zinc reacts rapidly and preferentially with the available oxygen, forming a slag of zinc oxide which is easily removed. Many alloys contain no zinc. They are described as zinc-free alloys and oxidation during melting is prevented by carrying out the procedure in an inert atmosphere.
The majority of alloy powders contain no mercury. Those products containing up to 3% mercury are called pre-amalgamated alloys. They are said to react more rapidly when mixed with mercury.
Fig. 21.1 This shows an occlusal amalgam filling which has been contoured and polished.
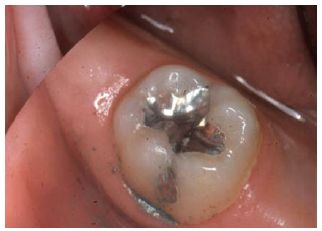
Table 21.1 Compositional limits of dental amalgam alloys specified in ISO 1559.
| Weight (%) | ||
| Metal | Limits prior to 1986 (‘conventional’ alloys) | Current limits |
| Silver | 65 (min) | 40 (min) |
| Tin | 29 (max) | 32 (max) |
| Copper | 6 (max) | 30 (max) |
| Zinc | 2 (max) | 2 (max) |
| Mercury | 3 (max) | 3 (max) |
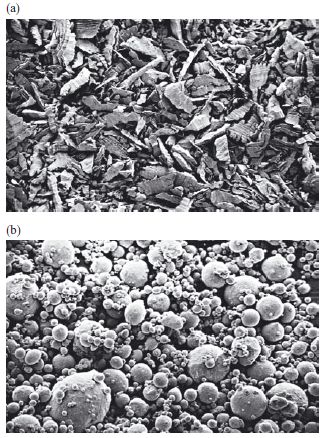
The shape and size of the alloy powder particles vary from one product to another. Two methods are commonly used to produce the particles. Firstly, filings of alloy may be cut from a prehomogenized ingot of alloy. These lathe-cut alloy powders are irregular in shape (Fig. 21.2a) and are graded according to size, being described as fine-grain or coarse-grain. Secondly, particles may be produced by atomization. Here, molten alloy is sprayed into a column filled with inert gas. The droplets of alloy solidify as they fall down the column. Particles produced in this way are either spherical or spheroidal in nature (Fig. 21.2b).
Lathe-cut alloys are normally subjected to two heat treating procedures. The first of these is a homogenization heat treatment (see Section 6.5) normally carried out on the alloy ingot before lathe-cutting and designed to produce homogeneous grains in which the Ag3Sn intermetallic compound predominates. During the formation of the ingot of alloy there is a tendency for phase separation to occur and for a cored grain structure to be formed. The heat treatment involves heating to about 420°C for several hours. The resulting alloy contains relatively large grains of γ phase material. The second heat treatment is carried out after lathe-cutting. This is a lower temperature treatment typically involving heating the alloy powder to approximately 100°C for about 1 hour. This treatment is referred to as alloy ageing; it is thought to remove residual stresses introduced during cutting and ensures that the alloy remains stable during future storage.
Fig. 21.2 Dental amalgam alloys. (a) Lathe-cut alloy particles (×100). (b) Spherical alloy particles (×500).
For spherical alloys the method of manufacture dictates that each small sphere is like an individual ingot. Thus homogenization is normally carried out for the reasons outlined above.
Many alloy powders are formulated by mixing particles of varying size or even shape in order to increase the packing efficiency of the alloy and reduce the amount of mercury required to produce a workable mix.
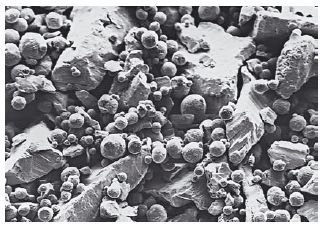
After the discovery in the 1960s that some of the properties of ‘conventional’ amalgam materials could be improved by the inclusion of great quantities of copper (in place of silver) a new class of materials was developed and became available for use by the dentist. The ISO Standard finally recognized this change in composition when the 1986 version of ISO 1559 was published. As shown in Table 21.1, these newer alloy powders have the same basic ingredients as the conventional products but they contain much greater concentrations of copper, typically 10–30% compared with less than 6% in the conventional materials. These newer alloys are referred to as copper-enriched alloys. In addition to the increased copper levels some alloys also contain small quantities of other metals such as palladium. Higher copper levels in alloy powders may be produced by the manufacturer in one of several ways. Lathecut, spherical or spheroidal powders can be produced in which the manufacturer alters the ratio of metals at the melting stage. Hence the resulting alloy particles are similar in shape and size to conventional alloys but simply contain a higher copper content. These are single-composition, copper-enriched alloys. An alternative approach is to blend particles of conventional alloy with those of, for example, a silver–copper alloy in order to achieve a higher overall copper content. Such blends are called dispersion-modified, copperenriched alloys and one widely used product contains two parts by weight of a lathe-cut alloy of conventional composition (less than 6% copper) and one part by weight of spherical silver–copper eutectic particles (Fig. 21.3). The latter particles contain 72 parts silver and 28 parts copper and the overall copper content in the blended alloy is 12%.
21.3 Setting reactions
The reaction which takes place when alloy powder and mercury are mixed is complex. Mercury diffuses into the alloy particles; very small particles may become totally dissolved in mercury. The alloy structure of the surface layers is broken down and the constituent metals undergo amalgamation with mercury. The reaction products crystallize to give new phases in the set amalgam. A considerable quantity of the initial alloy remains unreacted at the completion of setting. The structure of the set material is such that the unreacted cores of alloy particles remain embedded in a matrix of reaction products.
Fig. 21.3 Dispersion-modified alloy powder. Lathe-cut particles of conventional alloy and spherical particles of silver-copper eutectic alloy (×500).
In simplified terms, the reaction for conventional amalgam alloys may be given by the following unbalanced equation:
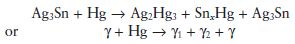
The primary reaction products are a silver–mercury phase (the γ1 phase) and a tin–mercury phase (the γ2 phase). The γ2 phase has a rather imprecise structure and the value of x in the formula SnxHg may vary from seven to eight. The equation emphasizes the fact that considerable quantities of unreacted alloy (γ phase) remain unconsumed.
For copper-enriched alloys the reaction may be represented by:
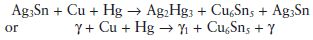
The essential difference between this and the reaction for conventional alloys is the replacement of the tin–mercury, γ2 phase in the reaction product with a copper–tin phase. The copper–tin phase may exist in the form of Cu6 Sn5 (η phase) or Cu3 Sn (ε phase) depending on the precise formulation of the alloy. In either case, the elimination of the γ2 phase has a profound effect on the properties of the set material.
In the case of the dispersion-modified, copper-enriched materials, it is believed that the particles of conventional lathe-cut alloy initially react to form γ1 and γ2 phases. The γ2 phase then reacts with copper from the silver–copper eutectic spheres to form the copper-tin phase. Thus, in these materials, the γ2 phase exists as an intermediate reaction product for a short time during setting. The reaction rate is quite slow and sometimes takes several days or even weeks to reach completion. This is reflected in the rate of development of mechanical properties.
21.4 Properties
Some of the important physical and mechanical properties of amalgam are specified as tests and requirements in the ISO specification for dental amalgam alloy (ISO 1559). The requirements are given in Table 21.2.
Dimensional changes: The setting reaction for amalgam involves a dimensional change. If cylindrical specimens of material are prepared and allowed to set in unrestrained conditions, plots of dimensional change versus time are akin to those shown in Fig. 21.4. Curves (a) and (b) are typical of results obtained for commonly used materials. A small contraction takes place during the first half hour or so. This corresponds to the stage during which mercury is still diffusing into the alloy particles. The upturn in the curve begins when crystallization of new phases becomes the predominant feature of the setting reaction. The outward thrust of growing crystals causes the expansion. The overall effect may cause a slight final expansion as shown in curve (a) or a slight final contraction as in curve (b). Factors which affect the amount of expansion of contraction include the type of alloy used, the particle size and shape and, most significantly, manipulative variables such as the pressure used to condense the amalgam into the cavity. It is important that the final set filling should not have dimensions which are very different from that of the cavity. A large contraction would result in a marginal-gap down which fluids could penetrate. A large expansion may result in the material protruding from the surface of the cavity or even in the fracture of the tooth (see Fig. 21.5). Hence, standard specification tests for dental amalgam permit only a small expansion (typically 0.1% maximum) or a small contraction (typically 0.1% maximum).
Table 21.2 Physical and mechanical properties of dental amalgam specified in ISO 1559.
| Property | Required value |
| Dimensional change (%) | −0.1 to +0.2 |
| Compressive strength (MPa) | |
| at 1 hour | 50 (minimum) |
| at 24 hours | 300 (minimum) |
| Creep (%) | 3.0 (maximum) |
Fig. 21.4 Dimensional change versus time for dental amalgam. Measurements started soon after mixing. (a) and (b) Examples of normal behaviour. (c) Example of moisture-contaminated zinc containing material. (note: logarithmic time scale)
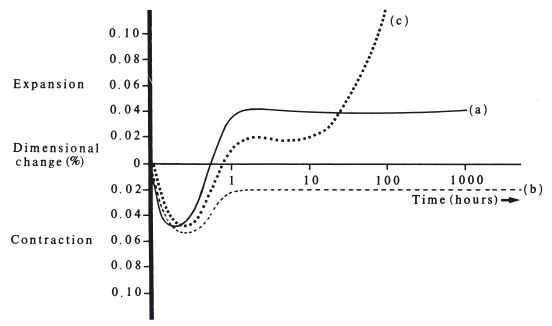
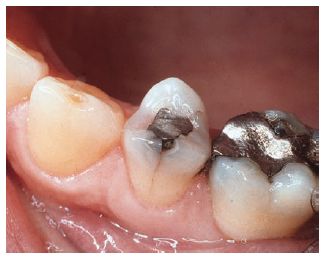
Fig. 21.5 An occlusal amalgam filling which has caused the tooth to crack. The most likely cause of this cracking is the expansion of the amalgam during or shortly after setting.
A far greater expansion than the maximum value given above may result if a zinc-containing amalgam is contaminated with moisture during condensation. Zinc reacts readily with water producing hydrogen:
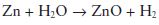
The liberation of hydrogen causes a considerable delayed expansion as illustrated by curve (c) in Fig. 21.4. This confirms the need for adequate moisture control when using these materials.
In order to facilitate a good marginal seal between amalgam fillings and the cavity wall it is suggested that a cavity varnish is used. Such varnishes consists of solutions of natural or synthetic resins in a volatile solvent such as ether. The varnish is applied to the cavity walls and after evaporation of the solvent a thin layer of resin covers the dentine. The amalgam is condensed against the varnish which helps to seal the cavity walls and to take up some of the strain if the amalgam expands. In order to effectively seal the cavity the varnish should be water resistant, a property which is not achieved in some of the natural resin varnishes.
Strength: The strength of dental amalgam is developed slowly. It may take up to 24 hours to reach a reasonably high value and continues to increase slightly for some time after that. At the time when the patient is dismissed from the surgery, typically some 15–20 minutes after placing the filling, the amalgam is relatively weak. It is necessary, therefore, to instruct patients not to apply undue stress to their freshly placed amalgam fillings. The requirements of the ISO Standard (Table 21.2) reflect the slow development of the strength which can occur with dental amalgam. The requirement for strength at 24 hours is six times the requirement at 1 hour.
Spherical particle alloys and copper-enriched alloys develop strength more rapidly than conventional lathe-cut materials. Fine-grain, lathe-cut products develop strength more rapidly than coarse-grain products (Fig. 21.6). There is little difference in the ultimate compressive strength values of the materials – all being adequate in this respect.
The tensile strength and transverse strength values of amalgam are very much lower than the compressive strength. The material is weak in thin sections and unsupported edges of amalgam are readily fractured under occlusal loads. Due regard must be paid to the mechanical properties of amalgam when considering cavity preparation. The material should be considered essentially brittle in nature, requiring adequate support from surrounding structures. Technique may play an important part in determining the final strength of amalgam. There is good correlation between strength and mercury content. Optimum properties are produced for amalgams containing 44–48% mercury. Since most materials are initially proportioned at more than 50% mercury it is necessary to reduce this level during manipulation.
Table 21.3 gives mechanical properties of a typical lathe-cut amalgam along with those of enamel a/>
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


