Implant assessment
Introduction
The implants are usually made of titanium and are described as either:
The Brånemark system
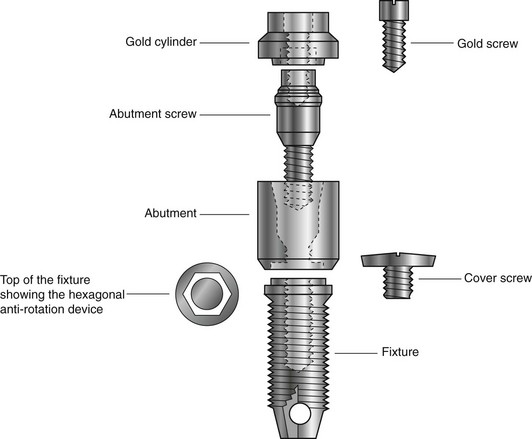
Treatment usually involves either a two-stage or a one-stage (non-submerged) surgical procedure followed by the restorative phase. Initially, in the two-stage technique the fixture is placed in vital bone ensuring a precision fit. The cover screw is screwed into the top of the fixture to prevent downgrowth of soft and hard tissue into the internal threaded area. The fixture is then left buried beneath the mucosa for 3–6 months. (It is important during this initial healing period to avoid loading the fixture although early loading protocols are being used in certain clinical circumstances.) The fixture is then surgically uncovered, the cover screw removed and the abutment (the transmucosal component) connected to the fixture by the abutment screw. An hexagonal anti-rotation device is incorporated into the top of the fixture. The gold cylinder, an integral part of the final restorative prosthesis, is finally connected to the abutment by the gold screw. A standard Brånemark implant is illustrated in Fig. 20.1.
Main indications
Replacement of missing teeth in patients with:
• Healthy dentitions that have suffered tooth loss because of trauma
• Developmentally missing teeth
• Remaining teeth not suitable as bridge abutments
• Severe ridge resorption making the wearing of dentures difficult
• Cleft palate and insufficient remaining teeth to support a denture/obturator
Treatment planning considerations
• The patient’s age, general health and motivation
• The condition and position of the remaining teeth (if present), including their occlusion
• The status of the periodontal tissues and the level of oral hygiene
• The condition – quality and quantity – of the edentulous mandibular or maxillary alveolar bone
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses