CHAPTER 20 Gingivitis and Periodontal Disease
The gingiva is the part of the oral mucous membrane that covers the alveolar processes and the cervical portions of the teeth. It has been divided traditionally into the free and the attached gingiva.1 The free gingiva is the tissue coronal to the bottom of the gingival sulcus. The attached gingiva extends apically from the free gingival groove to the mucogingival junction.
The gingival tissues are normally light pink, although the color may be related to the complexion of the person, the thickness of the tissue, and the degree of keratinization. The gingival color of the young child may be more reddish due to increased vascularity and thinner epithelium. The surface of the gingiva of a child appears less stippled or smoother than that of an adult. In the healthy adult, the marginal gingiva has a sharp, knifelike edge.2 During the period of tooth eruption in the child, however, the gingivae are thicker and have rounded margins due to the migration and cervical constriction of the primary teeth.
Delaney reports probing depths around primary teeth to be approximately 2 mm, with the facial and lingual probe sites shallower than the proximal sites.3 Children have a wider periodontal ligament than the adult. The width of the attached gingiva is narrower in the mandible than in the maxilla, and both widths increase with the transition from the primary to permanent dentition in the child. The alveolar bone surrounding the primary dentition demonstrates fewer trabeculae, less calcification, and larger marrow spaces.
Recent recognition that periodontal disease may have its origins in childhood has led dentists to be more aggressive in treatment. Studies confirm a high prevalence of gingival inflammation in children. Periodontal conditions that progress rapidly and result in the loss of primary and permanent teeth have been noted with increased frequency. Therefore, the American Academy of Pediatric Dentistry’s recommendations for children and adolescents includes placing greater emphasis on the prevention, early diagnosis, and treatment of gingival and periodontal disease in children.4 By establishing excellent oral hygiene habits in children, which will carry over to adulthood, the risk of periodontal disease is lowered.
Gingivitis is an inflammation involving only the gingival tissues next to the tooth. Microscopically, it is characterized by the presence of an inflammatory exudate and edema, some destruction of collagenous gingival fibers, and ulceration and proliferation of the epithelium facing the tooth and attaching the gingiva to it. Numerous studies indicate that marginal gingivitis is the most common form of periodontal disease and starts in early childhood.
Eastcott and Stallard have observed that plaque begins to form within 2 hours after the teeth are brushed.5 Coccal forms of bacteria form first on a thin fenestrated pellicle (organic bacteria-free film deposited on the tooth surface). The surface is completely covered with a smooth material 3 hours after brushing. Within 5 hours, plaque microcolonies develop, apparently by cell division. Between 6 and 12 hours, the covering material becomes thinner and is reduced to discontinuous small scattered areas. About 30% of the coccus bacteria are in various stages of division by 24 hours. Rod-shaped bacteria appear for the first time in 24-hour-old plaque. Within 48 hours the surface of the plaque is covered with a mass of rods and filaments.
Suomi and colleagues, in a study of approximately 1700 children 9 to 14 years of age, found that a relatively high percentage of children of all racial-ethnic groups had calculus (both supragingival and subgingival).6 From 56% to 85% of the children in the various age, sex, and racial-ethnic groups had supragingival calculus. The findings of this study indicate that most children 9 to 14 years of age who are of low socioeconomic status would benefit from inclusion in a preventive periodontal disease program based on improvement of oral hygiene.
ERUPTION GINGIVITIS
Weddell and Klein conducted a study to determine the prevalence of gingivitis in a group of children between 6 and 36 months of age.7 The children, patients of pediatricians in the Indianapolis area, had been born in the area, which has a fluoridated water supply. Among 299 white children, gingivitis was present in 13% of those 6 to 17 months of age, 34% of those in the 18- to 23-month age group, and 39% of those in the 24- to 36-month age group. Black children were not included in the study because of the inconsistency of their gingival colors. The gingivitis observed by Weddell and Klein was for the most part eruption gingivitis. Nevertheless, their findings support the view that an oral hygiene program should be initiated by parents when the child is very young.
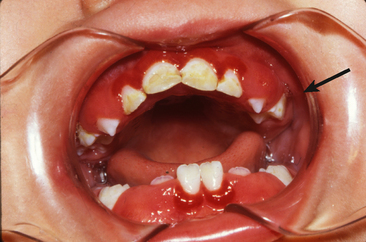
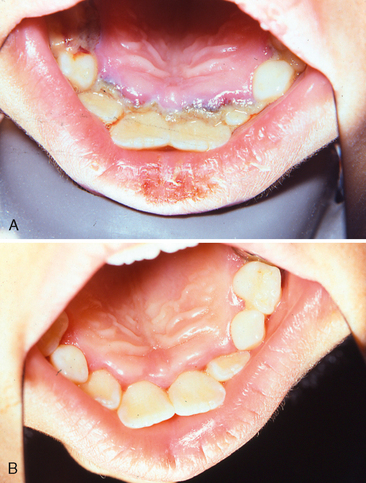
Food debris, materia alba, and bacterial plaque often collect around and beneath the free tissue, partially cover the crown of the erupting tooth, and cause the development of an inflammatory process (Fig. 20-1). This inflammation is most commonly associated with the eruption of the first and second permanent molars, and the condition can be painful and can develop into a pericoronitis or a pericoronal abscess. Mild eruption gingivitis requires no treatment other than improved oral hygiene. Painful pericoronitis may be helped when the area is irrigated with a counterirritant, such as Peroxyl (Colgate-Palmolive Co., New York, NY). Pericoronitis accompanied by swelling and lymph node involvement should be treated with antibiotic therapy.
DENTAL PLAQUE INDUCED GINGIVITIS
The degree of dental cleanliness and the condition of the gingival tissues in children are related. Horowitz and associates observed significant improvements in the gingivitis scores of schoolchildren after the initiation of a supervised daily plaque removal program.8 Children in grades 5 to 8 participated in the program, and successful results were maintained during three school years. The mean gingivitis scores were reduced 40% among girls and 17% among boys during the program period, whereas the children in the control group maintained essentially the same gingivitis scores for the period of the study. Adequate mouth hygiene and cleanliness of the teeth are related to frequency of brushing and the thoroughness with which bacterial plaque is removed from the teeth. Favorable occlusion and the chewing of coarse, detergent-type foods, such as raw carrots, celery, and apples, have a beneficial effect on oral cleanliness.
In a study of 2876 children residing in a naturally fluoridated area, Murray confirmed the high prevalence of gingivitis in the young population.9 He observed that inflammation of one or more papillae or margins associated with the incisor and canine teeth occurred in 90% of children 8 to 18 years of age. He, too, pointed to the importance of a good standard of oral cleanliness in reducing gingivitis and, ideally, preventing the progression of the disease in later life.
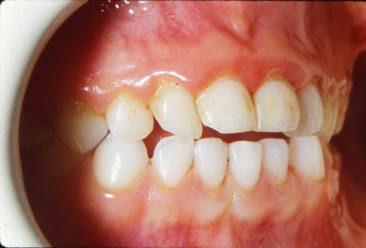
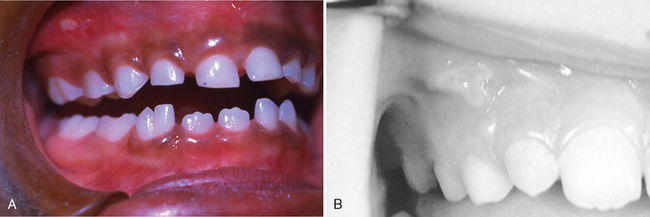
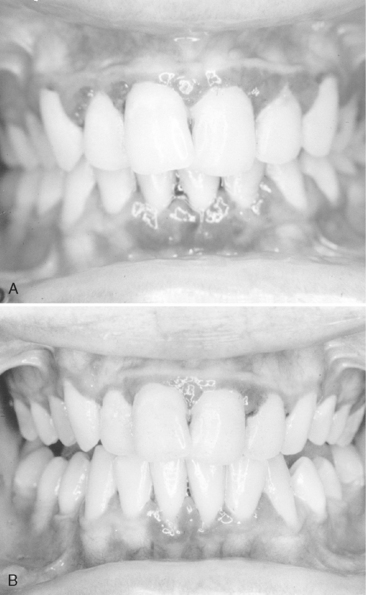
Gingivitis associated with poor oral hygiene is usually classified as early (slight), moderate, or advanced. Early gingivitis is quickly reversible and can be treated with a good oral prophylactic treatment and instruction in good toothbrushing and flossing techniques to keep the teeth free of bacterial plaque (Figs. 20-2 and 20-3). Gingivitis is generally less severe in children than in adults with similar plaque levels.
ALLERGY AND GINGIVAL INFLAMMATION
Matsson and Moller studied the degree of seasonal variation of gingival inflammation in children with allergies to birch pollen.10 Thirty-four allergic children were examined during two successive spring seasons and the one intervening fall. Age- and sex-matched controls were also examined in the fall. Gingival inflammation and the presence or absence of plaque were recorded, and a bleeding/plaque ratio was calculated for each subject. The results indicated an enhanced gingival inflammatory reaction in the allergic children during the pollen seasons. Although the authors acknowledge that the significance of gingival reaction during short allergic seasons is difficult to assess, they speculate that patients with complex allergies who have symptoms for longer periods may be at higher risk for more significant adverse periodontal changes.
ACUTE GINGIVAL DISEASE
HERPES SIMPLEX VIRUS INFECTION
Herpes virus causes one of the most widespread viral infections. The primary infection usually occurs in a child younger than 6 years of age who has had no contact with the type 1 herpes simplex virus (HSV-1) and who therefore has no neutralizing antibodies. It is believed that 99% of all primary infections are of the subclinical type. The infection may also occur in susceptible adults who have not had a primary infection (Fig. 20-4).
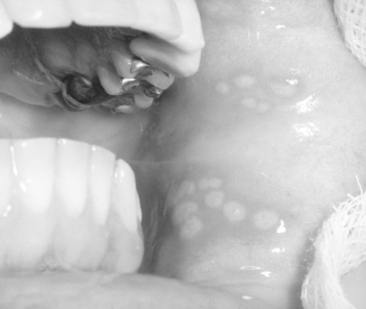
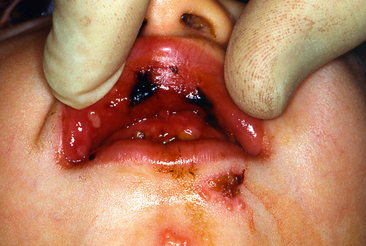
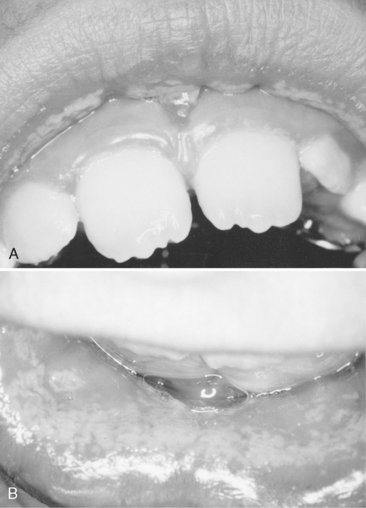
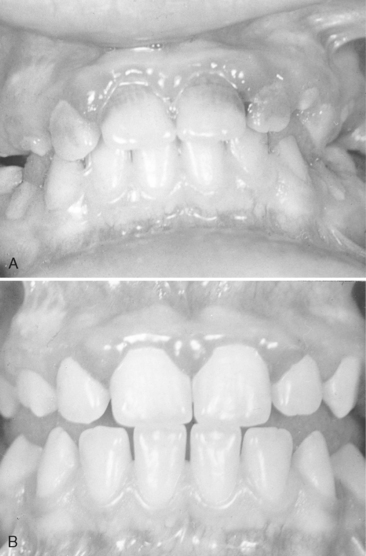
In some preschool children the primary infection may be characterized by only one or two mild sores on the oral mucous membranes, which may be of little concern to the child or may go unnoticed by the parents. In other children, the primary infection may be manifested by acute symptoms (acute herpetic gingivostomatitis). The active symptoms of the acute disease can occur in children with clean mouths and healthy oral tissues. In fact, these children seem to be as susceptible as those with poor oral hygiene. The symptoms of the disease develop suddenly and include, in addition to the fiery red gingival tissues, malaise, irritability, headache, and pain associated with the intake of food and liquids of acid content. A characteristic oral finding in the acute primary disease is the presence of yellow or white liquid-filled vesicles. In a few days the vesicles rupture and form painful ulcers, 1 to 3 mm in diameter, which are covered with a whitish gray membrane and have a circumscribed area of inflammation (Figs. 20-5 and 20-6). The ulcers may be observed on any area of the mucous membrane, including buccal mucosa, tongue, lips, hard and soft palate, and the tonsillar areas. Large ulcerated lesions may occasionally be observed on the palate or gingival tissues or in the region of the mucobuccal fold. This distribution makes the differential diagnosis more difficult. An additional diagnostic criterion is a fourfold rise of serum antibodies to HSV-1. The lesion culture also shows positive results for HSV-1.
Treatment of acute herpetic gingivostomatitis in children, which runs a course of 10 to 14 days, should include specific antiviral medication as well as provision for the relief of the acute symptoms so that fluid and nutritional intake can be maintained. The application of a mild topical anesthetic, such as dyclonine hydrochloride (0.5%) (Dyclone) before mealtime temporarily relieves the pain and allows the child to take in soft food. Another topical anesthetic, lidocaine (Xylocaine Viscous), can be prescribed for the child who can hold 1 teaspoon of the anesthetic in the mouth for 2 to 3 minutes and then expectorate the solution. Schaaf recommends as an alternative to the anesthetic a mixture of equal parts of diphenhydramine (Benadryl) elixir and Kaopectate.11 This material can be compounded by the pharmacist or mixed by the parent. The diphenhydramine has mild analgesic and antiinflammatory properties, whereas the kaolin-pectin compound coats the lesions. Because fruit juices are usually irritating to the ulcerated area, ingestion of a vitamin supplement during the course of the disease is indicated.
Although the treatments described may be useful, they are only palliative. The mainstay of definitive therapy is regular doses of specific systemic antiviral medication combined with systemic analgesics (acetaminophen or ibuprofen) during the course of the disease.12 The antiviral medications currently available are acyclovir, famciclovir, and valacyclovir. These medications inhibit viral replication in cells infected with the virus. Acyclovir (Zovirax; GlaxoSmithKline, Inc., Research Triangle Park, NC) should be administered in five daily doses to equal 1000 mg per day for 10 days. Acyclovir is available in capsules or suspension. Acyclovir therapy has been successfully used in infants and children.13 Famciclovir (Famvir; Novartis Pharmaceuticals Corporation, East Hanover, NJ) and valacyclovir (Valtrex; GlaxoSmithKline, Inc., Research Triangle Park, NC) are newer and possibly more effective antiviral agents, but their use in pediatric populations has not yet been studied. Food and Drug Administration (FDA) approved treatment for recurrent herpes simplex labialis (cold sore, fever blister) in children 12 years and older is valacyclovir 2 g, initially and 2 g 12 hours later. Famciclovir 1500 g one dose with prodrome (earliest sign of the lesions) is a FDA-approved treatment for herpes simplex labialis in adults, but it has not been studied in children. Bed rest and isolation from other children in the family are also recommended. Hale and colleagues reported an outbreak of herpes simplex infection in a group of 13 children occupying one floor in an orphanage.14 The children ranged in age from 11 to 35 months. In three of the children, a mild fever of brief duration and small oral lesions were the only signs of infection; these might easily have been unobserved in a situation different from that of an institutional study. The remaining children had symptoms of acute infection.
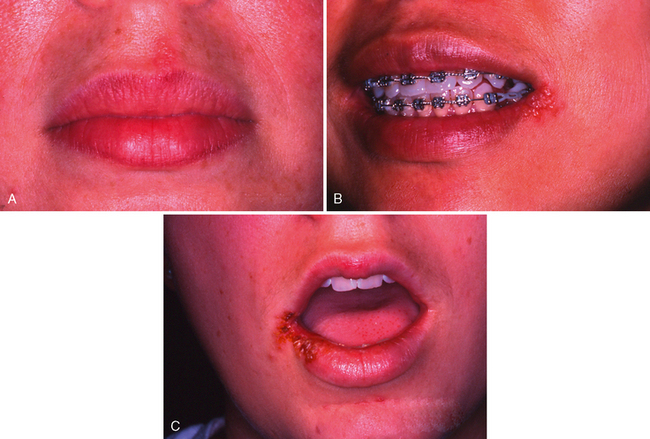
After the initial primary attack during early childhood, the herpes simplex virus becomes inactive and resides in sensory nerve ganglia. The virus often reappears later as the familiar cold sore or fever blister, usually on the outside of the lips (Fig. 20-7). Thus the disease has been commonly referred to as recurrent herpes labialis (RHL). However, approximately 5% of recurrences are intraoral. With the recurring attacks, the sores develop in essentially the same area. Kleinman and colleagues published the results of a national survey of 39,206 schoolchildren aged 5 to 17 years.15 A history of RHL was reported by 33% of the children.
The most effective treatment for these recurrences is the use of the specific systemic antiviral medications already discussed in connection with the treatment of the primary herpetic infection (acyclovir, famciclovir, and valacyclovir).12 The medication should be taken immediately after the prodromal symptom of recurrence. The daily dosages are the same as those for the primary infection, but the course of treatment is usually 5 days instead of 10. One-day therapy for recurrent herpes labialis is a total of 4 g valacyclovir given in a divided dose; 2 g initially with the prodrome, followed 12 hours later with another 2 g. This regimen has been approved for children 12 years of age and older. Another topical antiviral agent, penciclovir cream (Denavir; Novartis Consumer Health, Inc., Parsippany, NJ), may be applied to perioral lesions but should not be applied to intraoral lesions. The penciclovir cream and systemic antivirals should not be prescribed for concurrent use. The penciclovir cream can be applied every 2 hours while awake for 4 days, and it is approved for use in children 12 years of age and older. Topical 5% acyclovir cream may be prescribed for use five times daily for 4 days in children 12 years of age and older.
Other remedies for herpes simplex infection also include the amino acid lysine. The oral therapy is based on lysine’s antagonistic effect on another amino acid, arginine. Griffith and associates conducted an initial study in which 250 patients were given daily lysine doses of 1000 mg and were told to avoid eating arginine-rich foods, such as chocolate and nuts.16 The lysine therapy was continued until the patients had been lesion free for 6 months. l-Lysine monohydrochloride is available commercially in capsule form or tablets containing 100 or 312 mg of l-Lysine (General Nutrition Corp., Pittsburgh, Pa.). The patients reported that pain disappeared overnight in virtually every instance. New vesicles failed to appear, and a majority considered the resolution of the lesions to be more rapid than in the past. There was also a reduction in frequency of occurrences. Griffith and colleagues concluded that improper food selection may make adequate lysine intake precarious for some persons.16 Ingestion of cereals, seeds, nuts, and chocolate would produce a high arginine/lysine ratio and favor the development of herpetic lesions. Similar results are obtained when arginine is added to the medium in the laboratory to induce herpes proliferation. The avoidance of these foods, coupled with the selection of foods with adequate lysine, such as dairy products and yeast, should discourage herpes infection. These authors postulate that this may explain the low incidence of herpes in infants before they are weaned from a predominantly milk diet. Prophylactic lysine is apparently useful in managing selected cases of RHL if serum lysine is maintained at adequate concentrations.
Brooks and associates have reported that dentists are frequently exposed to HSV-1.17 They evaluated the risk of infection with the virus by assessing disease experience, comparing the individual’s history with the results of a complement fixation or antibody titration test, or both. Their study group consisted of 525 dental students, 94 dental faculty members, and 23 staff members. Although almost all of those with a history of herpetic infection showed antibodies to HSV-1, only 57% of those lacking such a history had neutralizing antibody titers of 1:10 or higher. This finding suggests that a significant portion of practicing dentists risk primary herpetic infection. Consequently, dentists and dental auxiliaries without a history of herpetic lesions might benefit from serologic testing. Considering the occupational disability that often accompanies HSV-1 infection of the finger or eye, effective barrier protection for health professionals is important.
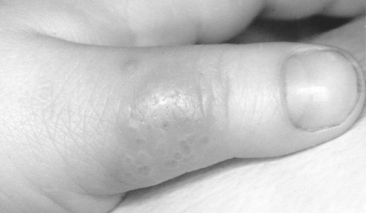
Primary herpetic infection has been observed on the dorsal surface of the thumb of a pediatric patient (Fig. 20-8). The child was a thumb sucker, and the acute primary infection was present in the mouth. The dorsal surface of the thumb, which rested on the lower incisor teeth, apparently became irritated, and an inoculation of the virus took place. The oral condition and the lesions on the thumb subsided in 2 weeks.
RECURRENT APHTHOUS ULCER (CANKER SORE)
The recurrent aphthous ulcer (RAU)—also referred to as recurrent aphthous stomatitis (RAS)—is a painful ulceration on the unattached mucous membrane that occurs in school-aged children and adults. The peak age for RAU is between 10 and 19 years of age. It has been reported to be the most common mucosal disorder in people of all ages and races in the world. This disorder, according to definitions adopted in the epidemiologic literature, is characterized by recurrent ulcerations on the moist mucous membranes of the mouth, in which both discrete and confluent lesions form rapidly in certain sites and feature a round to oval crateriform base, raised reddened margins, and pain. They may appear as attacks of minor or single, major or multiple, or herpetiform lesions. They may or may not be associated with ulcerative lesions elsewhere.18 In the national survey reported by Kleinman and colleagues, a history of RAU was reported by 37% of the schoolchildren and occurred approximately three times more often in white than in black schoolchildren.15 Ship and colleagues reported the prevalence estimates of RAU to range between 2% and 50% with most estimates between 5% and 25% (among medical and dental students, estimated prevalence is between 50% and 60%).19 Lesions persist for 4 to 12 days and heal uneventfully, leaving scars only rarely and only in cases of unusually large lesions. The description of RAU frequently includes the term canker sores (Fig. 20-9). The major form (RAS) is less common and has been referred to as periadenitis mucosa necrotica recurrens and Sutton disease. RAS has been associated with other systemic diseases: PFAPA (periodic fever, aphthous stomatitis, pharyngitis, adenitis), Behçet disease, Crohn disease, ulcerative colitis, celiac disease, neutropenia, immunodeficiency syndromes, Reiter syndrome, systemic lupus erythematosus, and MAGIC (mouth and genital ulcers with inflamed cartilage) syndrome.
The cause of RAU is unknown. Local and systemic conditions along with a genetic predisposition, as well as immunologic and infectious microbial factors, have been identified as potential causes. The condition may be caused by a delayed hypersensitivity to the L form of Streptococcus sanguis, which is a common constituent of the normal oral microbiota of humans. It is also possible that the lesions are caused by an autoimmune reaction of the oral epithelium. Epidemiologic studies by Ship and colleagues provide evidence for this hypothesis.19 These data indicate that both RHL and RAU may be produced by the same mechanism, despite the known infectious agent of RHL and the absence of any known virus for RAU. Scully and Porter reported strong associations with interleuken genotypes.18
Local factors include trauma, allergy to toothpaste constituents (sodium lauryl sulfate), and salivary gland dysfunction. In a review of the clinical problem, Antoon and Miller suggested that minor trauma is a common precipitating factor accounting for as many as 75% of the episodes.20 Injuries caused by cheek biting and minor facial irritations are probably the most common precipitating factors. Nutritional deficiencies are found in 20% of persons with aphthous ulcers. The clinically detectable deficiencies include deficiencies of iron, vitamin B12, and folic acid. While screening patients with aphthous ulcers, Wray and associates observed a history of unusually high incidence of gastrointestinal disorders.21 Stress may prove to be an important precipitating factor, particularly in stress-prone groups, such as students in professional schools and military personnel.
According to Greenspan and colleagues, either nonspecific factors (trauma, food allergy) or specific factors (bacterial or viral infection) may trigger a temporary imbalance in various cell subpopulations.22 This imbalance could then upset immune regulation and result in local destruction of the oral epithelium and thus ulceration. Ship and colleagues also suggested herpes simplex virus, human herpesvirus type 6, cytomegalovirus, Epstein-Barr virus, and varicella-zoster virus as possible causes of RAS.19
A variety of treatments have been recommended for RAU, but a completely successful therapy has not been found. Topical antiinflammatory and analgesic medicines and/or systemic immunomodulating and immunosuppression agents have been used for RAU. The primary line of treatment uses topical gels, creams, and ointments as antiinflammatory agents. Currently, a topical corticosteroid (e.g., 0.5% fluocinonide, 0.025% triamcinolone, 0.5% clobetasol) is applied to the area with a mucosal adherent (e.g., isobutyl cyanoacrylate, Orabase). For example, the application of triamcinolone acetonide (Kenalog in Orabase) to the surface of the lesions before meals and before sleeping may also be helpful. Binnie and colleagues reported that an antiinflammatory and antiallergic medication in the form of a topical paste is effective in reducing pain and accelerating healing of RAU ulcers.23 The active ingredient in the paste is 5% amlexanox and it is available under the trade name Aphthasol (Access Pharmaceuticals, Dallas, Texas). The paste is applied to the ulcer four times daily, after meals and at bedtime, until the ulcer heals. Zilactin (Zila Pharmaceuticals, Phoenix, Ariz), a topical paste with hydroxypropyl cellulose film, has also been used to adhere to the mucosa and cover the ulcer while providing pain relief for an extended period of time. Occlusive topical 2-otylcyanoacrylate adheres for 6 hours. Aloe vera freeze-dried gel extract adheres and forms an occlusive protective patch. In severe cases, oral prednisone has been prescribed.
Topical rinses have also been helpful for relief of RAU. Sucralfate has proved useful by coating the area. The topical application of tetracycline to the ulcers is often helpful in reducing the pain and in shortening the course of the disease. A mouthwash containing suspension of one of the tetracyclines has been helpful to some, but the mouthwash should not be swallowed. Chlorhexidine mouthwash has also been known to alleviate the symptoms of RAU. Swished dexamethasone elixir is useful to treat ulcerations in areas of the mouth that are difficult to access. Other findings that may be of significance include those of the two following studies: Meiller and associates have reported that the duration and severity of RAU lesions can be significantly reduced by vigorous twice-daily rinsing with an antimicrobial mouthwash (Listerine Antiseptic, Pfizer Warner Lambert Division, Morris Plains, NJ).24 Pedersen reported that some of her recent work suggests that varicella-zoster virus may be of etiologic importance in RAU.25 Furthermore, she found that six of eight patients with chronic severe RAU who were treated with acyclovir responded favorably within 2 days.
ACUTE NECROTIZING ULCERATIVE GINGIVITIS (VINCENT INFECTION)
The infectious disease commonly referred to as acute necrotizing ulcerative gingivitis (ANUG) is rare among preschool children in the United States, occurs occasionally in children 6 to 12 years old, and is common in young adults. Reade reported one instance of the typical picture of ulcerative gingivitis in a 15-month-old infant.26 Treponema vincentii and gram-negative fusiform bacilli were demonstrated in a smear. Recovery occurred within 36 hours after initiation of penicillin therapy and the application of hydrogen peroxide.
ANUG can be easily diagnosed because of the involvement of the interproximal papillae and the presence of a pseudomembranous necrotic covering of the marginal tissue (Fig. 20-10). Two microorganisms, Borrelia vincentii and fusiform bacilli, referred to as spirochetal organisms, are generally believed to be responsible for the disease. The clinical manifestations of the disease include inflamed, painful, bleeding gingival tissue, poor appetite, temperature as high as 40°C (104°F), general malaise, and a fetid odor.
There should be no difficulty in distinguishing ANUG from acute herpetic gingivostomatitis, although the two are sometimes confused. Round ulcers with red areolae on the lips and cheeks are characteristic of herpetic gingivostomatitis. Therapeutic prophylaxis and débridement bring about a favorable response in cases of ANUG but not in acute herpetic gingivostomatitis. A therapeutic trial of antibiotics reduces the acute symptoms in ANUG but not in the viral infection. Acute herpetic gingivostomatitis is most frequently seen in preschool children, and its onset is rapid. As stated earlier, ANUG rarely occurs in the preschool-aged group and develops over a longer period, usually in a mouth in which irritants and poor oral hygiene are present. On the other hand, acute oral infections initially diagnosed as ANUG have frequently been found later to be an oral manifestation of one of the xanthomatoses. The early stages of conditions such as Hand-Schüller-Christian disease and Letterer-Siwe disease are associated with many of the symptoms of ANUG.
ACUTE CANDIDIASIS (THRUSH, CANDIDOSIS, MONILIASIS)
Candida (Monilia) albicans is a common inhabitant of the oral cavity but may multiply rapidly and cause a pathogenic state when host resistance is lowered. Young children sometimes develop thrush after local antibiotic therapy, which allows the fungus to proliferate. The lesions of the oral disease appear as raised, furry, white patches, which can be removed easily to produce a bleeding underlying surface (Fig. 20-11). Neonatal candidiasis, contracted during passage through the vagina and erupting clinically during the first 2 weeks of life, is a common occurrence. This infection is also common in immunosuppressed patients (see Chapter 24).
ACUTE BACTERIAL INFECTIONS
The prevalence of acute bacterial infection in the oral cavity is unknown. Blake and Trott reported acute streptococcal gingivitis with painful, vivid red gingivae that bled easily.27 The papillae had enlarged, and gingival abscesses had developed. Cultures showed a predominance of hemolytic streptococci. Acute infections of this type may be more common than was previously realized. Littner and associates reported five cases, all in adults between 20 and 27 years of age.28 The diagnosis is difficult to make, however, without extensive laboratory tests. Broad-spectrum antibiotics are recommended if the infection is believed to be bacterial in origin. Improved oral hygiene is important in treating the infection. As with any acute microbial oral infection, chlorhexidine mouth rinses are also appropriate. The placement of dental restorations to restore adequate function and contour after the reduction of acute symptoms is equally important.
CHRONIC NONSPECIFIC GINGIVITIS
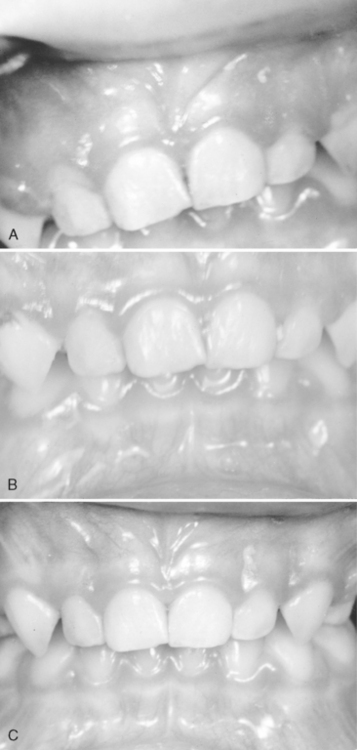
A type of gingivitis commonly seen during the preteenage and teenage years is often referred to as chronic nonspecific gingivitis. The chronic gingival inflammation may be localized to the anterior region, or it may be more generalized. Although the condition is rarely painful, it may persist for long periods without much improvement (Fig. 20-12).
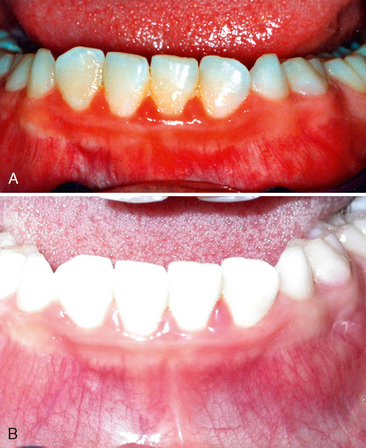
Glauser and colleagues observed an unusual gingivitis in Navajo Indians between 12 and 18 years of age, similar to that seen in Fig. 20-13, in which the fiery red gingival lesion is not accompanied by enlarged interdental labial papillae or closely associated with local irritants.29 The gingivitis showed little improvement after a prophylactic treatment. The age of the patients involved and the prevalence of the disease in girls suggested a hormonal imbalance as a possible factor. Histologic examination of tissue sections and the use of special stains ruled out a bacterial infection. Inadequate oral hygiene, which allows food impaction and the accumulation of materia alba and bacterial plaque, is undoubtedly the major cause of this chronic type of gingivitis.
Kaslick and colleagues studied 238 young adults and looked for a correlation between their periodontal status and their ABO blood type.30 They discovered that the chronic gingivitis group had a larger percentage of AB blood types and a smaller percentage of O blood type than the control group.
The cause of gingivitis is complex and is considered to be based on a multitude of local and systemic factors. Because dietary inadequacies are often found in the preteenage and teenage groups, the 7-day diet survey described in Chapter 10 is an important diagnostic aid. Insufficient quantities of fruits and vegetables in the diet, leading to a subclinical vitamin deficiency, may be an important predisposing factor. An improved dietary intake of vitamins and the use of multiple-vitamin supplements will improve the gingival condition in many children.
CHLORHEXIDINE AS A THERAPEUTIC PLAQUE CONTROL AGENT
Löe and Schiött reported highly significant inhibition of plaque formation and the prevention of gingivitis with use of an aqueous solution of 0.2% CH digluconate as a mouth rinse twice daily with swishing for 1 minute.31 Yankell and associates have shown that dental stain from CH mouth rinse can be significantly reduced with regular use of a tartar-control dentifrice.32
It is important to recognize that the beneficial use of CH as a therapeutic mouth rinse should be considered adjunctive to the practice of sound conventional plaque control measures as presented in Chapter 11 and elsewhere in this text. A study by Brecx and colleagues regarding the efficacy of Listerine, Meridol, and chlorhexidine (CH) mouth rinses found CH to be the most effective to supplement habitual mechanical oral hygiene.33 They also found a combination of habitual self-performed and nonsupervised oral hygiene with Listerine or Meridol is more beneficial for plaque control than the use of mechanical oral hygiene alone. Its adjunctive use would also seem most appropriate for therapy in cases in which attaining adequate plaque control is more difficult, such as during illness or convalescence after serious injuries.
The rationale to include use of daily antimicrobial mouth rinse to the child and adolescent’s oral hygiene regimens when inadequate plaque control exists to control and prevent periodontal disease and deliver antimicrobial agents to mucosal sites harboring bacteria throughout the mouth thereby complementing plaque control is widely accepted.34,35
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses