CHAPTER 16 Dental Materials
The website of the ADA (http://www.ADA.org) should be consulted for listings of materials that meet the ADA specifications. From that list the dentist should be able to select a brand that provides the desired manipulative characteristics.
In a like manner to the ADA, the Fédération Dentaire Internationale has been instrumental in the development of international specifications under the auspices of the International Standards Organization (ISO). The dental materials market has become international. The Medical Devices Amendments of 1976 to the Food and Drug Act gave the U.S. Food and Drug Administration (FDA) regulatory authority to protect the public from hazardous or ineffective medical and dental devices. Some dental products with claims for therapeutic effects (e.g., fluoride products) are considered drugs, but most dental materials used professionally are considered devices and are subject to regulation by the FDA Bureau of Medical Devices. Also included are over-the-counter dental products sold to the public, such as floss and denture adhesives.
MICROLEAKAGE AND BIOLOGIC CONSIDERATIONS
However, tooth structure possesses numerous undesirable characteristics as a substrate for bonding of an adhesive. It is rough, inhomogeneous in composition, covered with a tenacious layer of surface debris, and wet. These factors discourage adhesion. Furthermore, the reactivity (surface energy) of enamel is low, and therefore the surface does not easily attract other molecules to it.
AMALGAM
MECHANICAL AMALGAMATORS
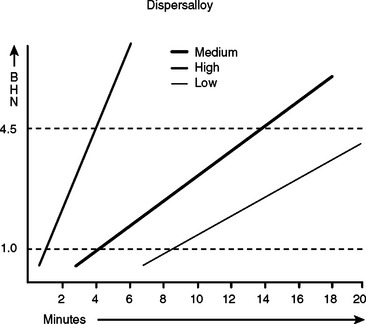
When first introduced, mechanical amalgamators for dental amalgam operated at a single speed that was usually below 3000 cpm. High-copper alloys in prefilled, self-activating capsules are designed for shorter trituration times at higher trituration speeds. Failure to activate these capsules reliably results in undertrituration and is a common problem with the use of older single-speed amalgamators. Because amalgamators also deteriorate with time, replacement of an older unit with a new high-speed amalgamator is desirable. A unit that allows multiple speeds of operation should be selected, because numerous other products such as dental cements are now marketed in capsules to be mixed in a dental amalgamator. The trituration times suggested by the amalgam alloy supplier are starting points. Amalgamators may vary in operating speed even within the same brand, and a unit’s performance may vary with line voltage or the number of times it is used in rapid succession. Trituration speed, as well as time, significantly influences the rate at which some amalgams harden(Fig. 16-1).
MARGINAL BREAKDOWN AND BULK FRACTURE
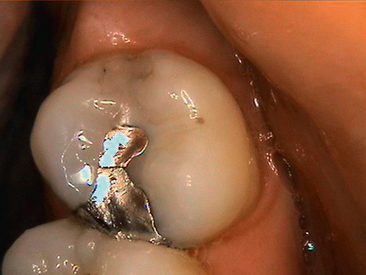
Bulk fracture of amalgam is much less common with high-copper amalgam alloys. Those cases that do occur likely have one of two causes. Poor cavity design resulting in an insufficient bulk of material across the isthmus can lead to failure of even a high-strength alloy, as illustrated in Fig. 16-2. The other reason for bulk fracture is premature loading of the restoration. Unlike a resin matrix composite, amalgam gains strength slowly over the first 24 hours. Premature loading can result in minute fractures that are not apparent for weeks or even months. The use of a rapid-setting amalgam with a high 1-hour compressive strength should be considered when treating a pediatric patient in whom compliance with instructions to refrain from biting down hard on the freshly placed amalgam is in question.
BONDED AMALGAM RESTORATIONS
Because dental amalgam does not adhere to tooth structure, it must be retained mechanically by the design of the cavity preparation and/or mechanical devices such as pins. The placement of an amalgam does not strengthen the compromised remaining tooth structure and subsequent fracture may occur, particularly in molar teeth with relatively large mesiodistocclusal amalgam restorations. The use of dental adhesive systems, as described in detail in the section related to resin composites, as lining materials for amalgam to create a “bonded amalgam restoration” has been suggested. Several products are marketed specifically for this purpose. In general, they are chemically activated dentin-bonding systems over which the amalgam is condensed before the resin adhesive has hardened. This results in an intermixing of the unset resin and the plastic amalgam at the interface and forms a mechanical bond as both materials harden. It is important to distinguish this application from the use of a dental adhesive to seal the dentin surface and reduce early microleakage as previously discussed. When dental adhesives are used to seal the dentin surface, the adhesive should be polymerized before the amalgam is placed. Bond strengths reported in laboratory studies between amalgam and dentin are lower than the maximum reported for resin composite bonded to dentin. In vitro studies also show that teeth restored with bonded amalgams are more resistant to fracture than those in which amalgam is placed without a bonding adhesive.
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses