CHAPTER 10 Dental Caries in the Child and Adolescent
We know that good oral health is an integral component of good general health. Although enjoying good oral health includes more than just having healthy teeth, many children have inadequate oral and general health because of active and uncontrolled dental caries. According to the first-ever United States Surgeon General’s report on oral health in America published in May 2000, dental caries is the single most common chronic childhood disease.1 Dental caries is five times more common than asthma and seven times more common than hay fever. Furthermore, as Edelstein and Douglass noted, dental caries is not self-limiting, like the common cold, nor amenable to treatment with a simple course of antibiotics, like an ear infection.2 After analyzing the National Health Interview Survey data from 1993 through 1996, Newacheck and colleagues concluded that dental care is the most prevalent unmet health need among American children.3 Much other available data verify that we learned a great deal during the twentieth century about preventing dental caries, but other variables that contribute to the spread of the disease among many people in the world continue to thwart our efforts to eliminate this major health problem. Although effective methods are known for prevention and management of the disease, the unmet need for treatment, especially in children, does not seem to be diminishing. Gift has estimated that 51 million school hours per year are lost in the United States because of dental-related illness.4
The National Institutes of Health sponsored a Consensus Development Conference on Diagnosis and Management of Dental Caries Throughout Life in March 2001.5 Thirty-four papers were presented by recognized experts on dental caries. The October 2001 issue of the Journal of Dental Education published the entire proceedings of that conference. The complete journal issue provides many good updates on the diagnosis and management of dental caries. A few of the papers from the conference are cited in this chapter.
ETIOLOGY OF DENTAL CARIES
For as long as the science of dentistry has existed, there has been theorizing about the cause of dental caries. Today, all experts on dental caries generally agree that it is an infectious and communicable disease and that multiple factors influence the initiation and progression of the disease. The disease is recognized to require a host (tooth in the oral environment), a dietary substrate, and aciduric bacteria.6 The saliva (also considered a host component), the substrate, and the bacteria form a biofilm (plaque) that adheres to the tooth surface. Over time the presence of the substrate serves as a nutrient for the bacteria, and the bacteria produce acids that can demineralize the tooth. The flow, dilution, buffering, and remineralizing capacity of saliva are also recognized to be critical factors that affect, and in some ways regulate, the progression and regression of the disease. If the oral environment is balanced and favorable, saliva can contribute to strengthening of the tooth by supplying the components known to help build strong apatite structure. If the oral environment is unfavorable (too much acid is produced too often), an adequate flow of saliva can help dilute and buffer the acid, and thus slow the rate of damage to the tooth or even repair it. The critical pH for dissolution of enamel has been shown to be about 5.5. Once the process reaches dentin, dissolution can occur at a considerably higher pH. In addition, we know of many anatomic, behavioral, dietary, genetic, social, cultural, socioeconomic, and therapeutic variables that can significantly influence the level of caries activity favorably or unfavorably.
Studies by Orland7 and by Fitzgerald and colleagues8 demonstrated that dental caries do not occur in the absence of microorganisms. Animals maintained in a germ-free environment did not develop caries even when fed a high-carbohydrate diet. However, dental caries did develop in these animals when they were inoculated with microorganisms from caries-active animals and then fed cariogenic diets.
A number of microorganisms can produce enough acid to demineralize tooth structure, particularly aciduric streptococci, lactobacilli, diphtheroids, yeasts, staphylococci, and certain strains of sarcinae. Streptococcus mutans has been implicated as one of the major and most virulent of the caries-producing organisms. Consequently S. mutans has been targeted in a large share of research. Loesche conducted an extensive review of the literature regarding the etiology of caries.9 He concluded that the evidence suggests that S. mutans, possibly Streptococcus sobrinus, and lactobacilli are human odontopathogens. He stated that aciduricity appears to be the most consistent attribute of S. mutans and is associated with its cariogenicity. He also observed that other aciduric species such as S. sobrinus may be more important in smooth-surface decay and are perhaps associated with rampant caries. Loesche concluded the review with the suggestion that treatment strategies that interfere with the colonization of S. mutans may have a profound effect on the incidence of caries in humans.9 As caries research proceeds, there seems to be increasing evidence that disease may result from a group of microbial species in the tooth-adhering biofilm. It is not clear which combinations of organisms are most blameworthy.
Wan and colleagues have published a series of three papers that report on 111 infants whom they observed to 2 years of age. They found S. mutans colonization in infants as young as 3 months, and more than 50% of the predentate infants were infected by 6 months of age. By 24 months of age, 84% of the children harbored the bacteria.10–12 Investigations by Davey and Rogers13 and by Berkowitz and Jones14 have confirmed that S. mutans is transmitted orally from mother to infant, whereas Brown and colleagues15 have demonstrated a relationship between the numbers of S. mutans present in mothers and infants. Research by Kohler and colleagues demonstrated that reducing the numbers of oral S. mutans in mothers delayed the colonization of the organisms in the mouths of their children.16 Their findings also showed that 52% of the children who carried S. mutans at 3 years of age had caries, whereas only 3% of the children without demonstrable S. mutans had caries at the same age. In 1988, these same investigators reported that, the earlier the colonization of S. mutans in the mouths of children, the higher the caries prevalence at 4 years of age.17 Caufield and colleagues suggested the possibility of a “window of infectivity” between 19 and 33 months of age during which most children acquire the cariogenic organisms.18 The mother was the most common source of transmission of the bacteria to the child. In a group of 122 children 6 to 24 months of age, Mohan and colleagues found oral mutans streptococci colonization in 20% of the children younger than 14 months of age.19 In addition, logistic regression models that controlled for both age and number of teeth indicated that children who consumed sweetened beverages in their baby bottle were four times more likely to have mutans streptococci than children who only consumed milk. Although investigations to elucidate how and when mutans streptococci are transmitted to children are continuing, essentially all experts agree that the earlier transmission occurs, the higher the caries risk.
The acids that initially demineralize the enamel have a pH of 5.5 to 5.2 or less and are formed in the plaque material, which has been described as an organic nitrogenous mass of microorganisms firmly attached to the tooth structure. This film, which exists primarily in the susceptible areas of the teeth, has received a great deal of attention. Considerable emphasis is currently being given to plaque and its relationship to oral disease. Methods of chemical plaque control are being investigated. The method that has received the most attention during the past decade is the use of antimicrobial agents whose action is selective against certain types of microorganisms, including S. mutans. Chlorhexidine and other agents are available in antimicrobial oral rinse solutions. Another approach involves the use of monomolecular layers on the tooth surface that prevent the adherence of microorganisms. Perhaps by learning how to make enamel resistant to bacterial colonization (plaque formation), both caries and gingival disease can be reduced.
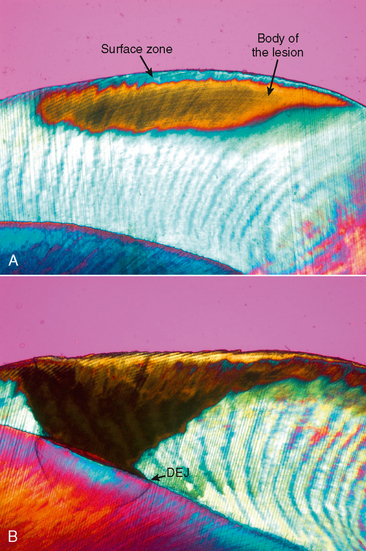
The acids involved in the initiation of the caries process are normal metabolic by-products of the microorganisms and are generated by the metabolism of carbohydrates. Because the outer surface of enamel is far more resistant to demineralization by acid than is the deeper portion of enamel, the greatest amount of demineralization occurs 10 to 15 mm beneath the enamel surface (Fig. 10-1). The continuation of this process results in the formation of an incipient subsurface enamel lesion that is first observed clinically as a so-called white spot. Unless the demineralization is arrested or reversed (remineralization), the subsurface lesion continues to enlarge, with the eventual collapse of the thin surface layer and the formation of a cavitated lesion.
CARIES PREVALENCE IN PRESCHOOL CHILDREN
Weddell and Klein examined 441 children who ranged in age from 6 to 36 months and resided in a community with water fluoridation.20 They found dental caries in 4.2% of the children 12 to 17 months of age, 19.8% of those 24 to 29 months of age, and 36.4% of those 30 to 36 months of age. Children in the middle and middle-low socioeconomic groups showed a trend toward higher caries frequencies. Edelstein and Tinanoff found that 30.5% of 200 preschool children had caries detectable by visual or radiographic examination.21 These children were recruited from a private pediatric dental office and ranged in age from 5 months to 5 years 11 months (mean, 3 years 8 months).
Douglass and colleagues determined the caries prevalence in children 3 to 4 years of age from a fluoridated community in Connecticut who were enrolled in the same Head Start program.22 They compared the caries prevalence in 517 children enrolled in 1999 with that in 311 children enrolled in 1991. They found a caries prevalence of 38% in the children enrolled in 1999 and 49% in those enrolled in 1991. They noted, however, that the children enrolled in 1999 had a greater severity of maxillary anterior caries. Tang and colleagues performed dental caries examinations on 5171 preschool children recruited from public health assistance programs in Arizona.23 They found caries in 6.4% of 1-year-olds, nearly 20% of 2-year-olds, 35% of 3-year-olds, and 49% of 4-year-old children in the study.
In general, other reports of caries prevalence among children in various parts of the world show rates that seem to be comparable to those cited here. Another common element of caries prevalence in the United States and throughout the world is that children from families in low socioeconomic groups consistently have greater caries prevalences than their peers from families at a higher socioeconomic level. Vargas and colleagues reported that 27.4% of a sample of 3889 children 2 to 5 years of age had at least one decayed or filled primary tooth.24 These children were part of a larger sample of individuals included in the third National Health and Nutrition Examination Survey (NHANES III, 1988-1994). This sample of children was 51.4% male and 48.6% female, with an ethnic distribution of 64.1% non-Hispanic white, 16.0% black, 9.5% Mexican American, and 10.4% other ethnicity. The family incomes for these children were distributed into four groups categorized from low to high, and these groups comprised 27.9%, 25.5%, 21.6%, and 24.9% of the sample, respectively.
In a longitudinal evaluation of caries patterns in 317 children followed an average of 7.8 years in private dental practices, Greenwell and colleagues made several noteworthy discoveries.25 They found that 84% of the children who were caries-free in the primary dentition remained caries-free in the mixed dentition. Children with pit and fissure caries in the primary dentition were more likely to develop smooth-surface caries of primary teeth than the caries-free children. Fifty-seven percent of the children with proximal lesions in primary molars in the primary dentition developed additional primary molar proximal lesions in the mixed dentition. Children with faciolingual decay (nursing caries) were at the highest risk of any group for developing additional carious lesions. These investigators also discovered levels of caries susceptibility in children that can be characterized as caries-free, pit and fissure caries, and proximal molar caries patterns.
CARIES PREVALENCE IN SCHOOL CHILDREN
The report by Vargas and colleagues provides additional representative data for school children as well.24 Their report revealed that 61% of the sample of children 6 to 12 years of age had at least one decayed or filled primary tooth. Furthermore, in the sample of 4116 children 6 to 14 years of age, 40% had at least one decayed or filled permanent tooth. Of the 1383 children 15 to 18 years of age, 89.8% had at least one decayed or filled permanent tooth. The ethnic and family income distributions for children in these different age groups were comparable to those outlined in detail for the preschool children. This information, along with that in many other published reports, clearly indicates that managing the disease of dental caries among children remains a formidable task despite the advances made in various preventive programs. Edelstein and Douglass have noted, “The popular statement that half of U.S. school children have never experienced tooth decay fails profoundly to reflect the extremity and severity of this still highly prevalent condition of childhood.”2
RAMPANT DENTAL CARIES
There is no complete agreement on the definition of rampant caries or on the clinical picture of this condition. It has been generally accepted, however, that the disease referred to as rampant caries is, in terms of human history, relatively new. Rampant caries has been defined by Massler as a “suddenly appearing, widespread, rapidly burrowing type of caries, resulting in early involvement of the pulp and affecting those teeth usually regarded as immune to ordinary decay.”26
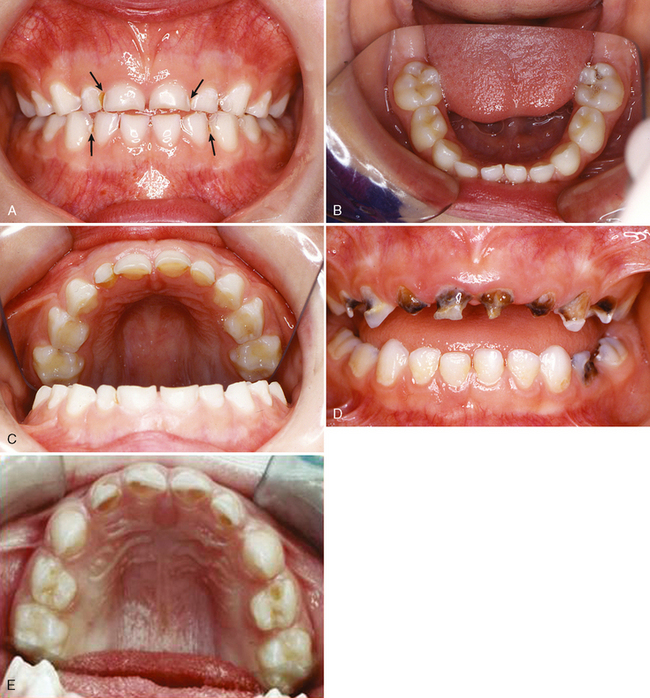
When a patient has what is considered an excessive amount of tooth decay, one must determine whether that person actually has a high susceptibility and truly rampant caries of sudden onset or whether the oral condition represents years of neglect and inadequate dental care. Young teenagers seem to be particularly susceptible to rampant caries, although it has been observed in both children and adults of all ages (Fig. 10-2).
There is considerable evidence that emotional disturbances may be a causative factor in some cases of rampant caries. Repressed emotions and fears, dissatisfaction with achievement, rebellion against a home situation, a feeling of inferiority, a traumatic school experience, and continuous general tension and anxiety have been observed in children and adults who have rampant dental caries. Because adolescence is often considered to be a time of difficult adjustment, the increased incidence of rampant caries in this age group lends support to this theory. An emotional disturbance may initiate an unusual craving for sweets or the habit of snacking, which in turn might influence the incidence of dental caries. On the other hand, a noticeable salivary deficiency is not an uncommon finding in tense, nervous, or disturbed persons. Indeed, various forms of stress in both children and adults, as well as various medications (e.g., tranquilizers and sedatives) that are commonly taken to help cope with stress, are associated with decreased salivary flow and decreased caries resistance caused by impaired remineralization. It is well known that radiation therapy to the head and neck often results in significantly diminished salivary function and may place patients at high risk for severe caries development.
EARLY CHILDHOOD CARIES, SEVERE EARLY CHILDHOOD CARIES, NURSING CARIES, BABY BOTTLE TOOTH DECAY
The American Academy of Pediatric Dentistry (AAPD) defines early childhood caries (ECC) as the presence of one or more decayed (noncavitated or cavitated), missing (as a result of caries), or filled tooth surfaces in any primary tooth in a child 71 months of age or younger. The AAPD also specifies that, in children younger than 3 years of age, any sign of smooth-surface caries is indicative of severe early childhood caries (S-ECC).27
For many years it has been recognized that, after eruption of the primary teeth begins, excessively frequent bottle feedings and/or prolonged bottle or breast-feeding is often associated with early and rampant caries. The clinical appearance of the teeth in S-ECC in a child 2, 3, or 4 years of age is typical and follows a definite pattern.28 There is early carious involvement of the maxillary anterior teeth, the maxillary and mandibular first primary molars, and sometimes the mandibular canines (Fig. 10-3). The mandibular incisors are usually unaffected. A discussion with the parents often reveals an inappropriate feeding pattern: the child has been put to bed at afternoon nap time and/or at night with a nursing bottle holding milk or a sugar-containing beverage. The child falls asleep, and the liquid becomes pooled around the teeth (the lower anterior teeth tend to be protected by the tongue). It would seem that the carbohydratecontaining liquid provides an excellent culture medium for acidogenic microorganisms. Salivary flow is also decreased during sleep, and clearance of the liquid from the oral cavity is slowed.
Gardner and colleagues reported four case histories in which the same pattern of caries was observed, and in each child the condition was attributed to a specific breast-feeding habit.29 In each case the mother explained that human milk was the main source of nutrition. The investigators recommend that from birth the infant should be held while feeding. The child who falls asleep while nursing should be burped and then placed in bed. In addition, the parent should start brushing the child’s teeth as soon as they erupt.
The AAPD endorses the policy statement of the American Academy of Pediatrics (AAP) on breast-feeding and the use of human milk.30 The AAP statement includes the acknowledgment that “breast-feeding ensures the best possible health as well as the best development and psychosocial outcomes for the infant.” However, both organizations discourage extended or excessive frequency of feeding times (from the breast or bottle) and encourage appropriate oral hygiene measures for infants and toddlers.
Dilley and colleagues observed a large number of children with prolonged nursing habit caries and concluded that there was no association between the nursing habit and family background, except that the families were predominantly from lower socioeconomic groups.31 All subjects demonstrated prolonged breast-feeding or bottle feeding, with milk reported to be the liquid most often used in the bottle. Parents indicated that they did not know when weaning should occur and when oral hygiene should be instituted. The authors also observed nearly symmetric caries patterns.
Hallonsten and colleagues screened 3,000 18-month-old children for dental caries and ongoing breastfeeding.32 Twelve (19.7%) of the 61 children still being breast-fed had caries, while 51 (1.7%) of the 2,939 children not being breast-fed had caries. The authors found that children who experience prolonged breast-feeding tend to develop unsuitable dietary habits that put them at risk for caries at an early age.
S-ECC may be prevented by early counseling of the parents. This is one reason for suggesting that children receive their first dental examination between 6 and 12 months of age when S-ECC is not likely to have developed. In a comprehensive report prepared for the Oral Health Subcommittee of the Healthy Mothers–Healthy Babies Coalition, Ripa states, “Priority needs to be given to a major national educational program directed toward educating the public about nursing caries.”33 The educational program must involve direct contact with pregnant women, parents, and other caregivers in population subgroups with a high prevalence of nursing caries.
ADDITIONAL FACTORS KNOWN TO INFLUENCE DENTAL CARIES
SALIVA
Salivary Deficiency
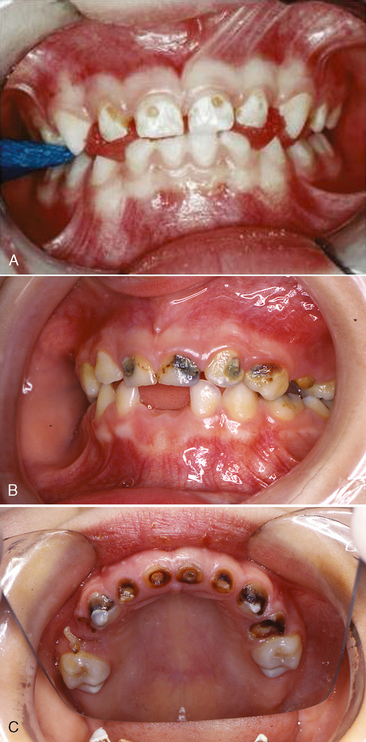
One of the first descriptions of a severe salivary deficiency with its deleterious effect on the dentition was reported by Hutchinson in 1888.34 Since that time, many reports have emphasized the importance of a normal flow of saliva in preventing a breakdown of the dentition. A reduction in the salivary flow may be temporary or permanent. When the quantity is only moderately reduced, the oral structures may appear normal. A pronounced reduction or complete absence of saliva, however, results in an acidic environment with rampant caries (Fig. 10-4). In addition to the rapid destruction of the teeth, there may be dryness and cracking of the lips, with fissuring at the corners of the mouth, burning and soreness of the mucous membranes, crusting of the tongue and palate, and sometimes paresthesia of the tongue or mucous membrane.
One study indicated that the minimum effective dose of many of the antihistaminic drugs can reduce salivary flow by as much as 50%.35 Dryness of the mouth may occur after the use of a variety of tranquilizers and antihistamines. It has likewise been observed that dry mouth and rampant caries may accompany a systemic condition, such as myasthenia gravis. In this disease, the acetylcholine that is necessary for the proper transmission of nerve impulses is destroyed; as a result, the salivary glands do not receive adequate stimulation.
Determination of Salivary Flow
If a patient has no known existing conditions that may cause hyposalivation and if the clinician notices a small pool of saliva in the floor of the mouth during oral examination, it is not unreasonable to assume that the patient has adequate salivary quantity and flow. Little information is available about salivary flow rates in children, but Crossner reported that in children 5 to 15 years of age, the rate of mixed whole stimulated saliva increases with age, and boys have consistently higher rates than girls.36 If inadequate salivary flow is known to exist or is suspected, measurement of salivary flow can provide a baseline useful for comparing with later measurements after implementation of adjunctive therapy.
To evaluate the adequacy of salivary flow, Zunt recommends establishing the unstimulated salivary flow (USF) rate.37 The USF rate is measured after a period of 1 hour without eating, drinking, chewing gum, or brushing the teeth. Sitting in the “coachman” position, on the edge of the dental chair, the patient passively drools into a funnel inserted into a graduated cylinder for 5 minutes. The eyes should remain open except for blinking during the 5-minute collection period. The head and neck should be bent, and the arms should rest comfortably on the thighs or knees. The volume of saliva collected in the cylinder after 5 minutes is divided by 5 to determine the USF. A USF rate of less than 0.1 mL per minute is diagnostic of salivary gland hypofunction. If the USF rate is less than 0.1 mL per minute, the next step is to measure the stimulated salivary flow (SSF). The patient should chew unflavored paraffin for 45 chews or 1 minute and expectorate into a funnel inserted into a graduated cylinder. The SSF rate should be 1 to 2 mL per minute; less than 0.5 mL per minute is scored as an abnormal rate. A convenient alternative method for measuring USF is the modified Schirmer technique, which uses a calibrated paper test strip to collect saliva in the floor of the mouth.
In patients who are known or suspected to have salivary deficiency, it is not unusual to find a salivary flow ranging from slightly below normal to practically a dry mouth. If there is a deficiency of saliva or a dry mouth, the cause should be sought. Sometimes the cause is readily determinable; sometimes it is obscure. An emotional disturbance should not be overlooked as a cause in a patient of any age. Psychotherapy may be helpful in these cases. If the cause cannot readily be determined, perhaps it should be assumed that the sparse flow is related to inadequacies in the diet, particularly a vitamin deficiency or excessive sugar consumption to the exclusion of needed foods. Monthly quantitative analyses of the saliva should be performed to determine whether dietary improvement is accompanied by an increased flow.
If the salivary glands have not undergone degenerative or metaplastic change and if the nerve pathways between the central nervous system and the salivary glands are still intact, salivary stimulants may be recommended. If dryness of the mouth is attributable to dehydration, increased fluid intake should be recommended. The use of gustatory stimulants (sugar-free candy) or masticatory stimulants (xylitol gum) has been suggested as an adjunct to encourage salivation. Prescription sialagogue medications, also known as secretagogues, such as pilocarpine and cevimeline may be of benefit in improving the salivary flow rate in patients with Sjögren syndrome or with radiation damage to salivary glands. The use of sialagogue medications has not been studied in pediatric populations, but these agents are considered safe for most adult patients and have been used successfully in older children. The use of salivary substitutes has been suggested by Shannon and colleagues as helpful in preventing soft tissue problems associated with dry mouth.38 Saliva substitutes, as well as fluoride and chlorhexidine rinses, are also reported to enhance remineralization and promote resistance to demineralization of tooth surfaces, and may help prevent radiationinduced caries.
Viscosity of Saliva
It has long been suggested that the viscosity of saliva is related to the rate of dental decay. Both thick, ropy saliva and thin, watery saliva have been blamed for rampant dental caries. Previous work has shown a statistically significant direct relationship between the viscosity of saliva and the number of decayed, missing, and filled teeth.39 This relationship held true for all members of the observation group, regardless of age. Patients with thick, ropy saliva invariably had poor oral hygiene. The teeth were covered with stain or plaque, and the rate of dental caries ranged from greater than average to rampant.
Children who consume excessive amounts of carbohydrates often have not only a sparse flow but also viscous saliva. Even minimal doses of some antihistaminic drugs will result in a greatly increased viscosity of saliva in some persons.35
Although relatively little information specific to salivary function, flow, and viscosity in children is available, an excellent review article by Leone and Oppenheim provides much additional information regarding the relationship between saliva and dental caries.40
SOCIOECONOMIC STATUS
The Surgeon General’s report of 2000 indicated that one in four American children is born into poverty.1 The report notes that children and adolescents living in poverty suffer twice as much tooth decay as their more affluent peers and their disease is more likely to go untreated. The report also mentioned that, although continuing reductions of dental caries in permanent teeth have been achieved, caries prevalence in primary teeth has stabilized or possibly increased in some population groups. A Census Bureau report published in March 2003 showed that the poverty rate for children in the United States rose in 2002, whereas it dropped for people 65 years and older.41 Nearly half of the 35 million people living in poverty were children. These are alarming data calling attention to a huge unmet oral health care need in the United States.
Following the report of the Surgeon General, Edelstein pointed out that, paradoxically, children living in poverty also have the highest rates of dental insurance coverage, largely through the Medicaid program and the State Children’s Health Insurance Program.42 Yet Medicaideligible children who have cavities have twice the number of carious teeth and twice the number of visits for pain relief but fewer total dental visits than children in families with higher incomes. He also noted that these disparities continue into adolescence and young adulthood but to a lesser degree. Because practitioners have the opportunity to assess the oral health of poor children individually, they will identify some patients at low risk for dental caries. However, the available data confirm that, from a demographic perspective, economically poor children are at high risk for dental caries.
ARRANGEMENT OF THE TEETH IN THE ARCH
Crowded and irregular teeth are not readily cleansed during the natural masticatory process. It is likewise difficult for the patient to clean the mouth properly with a toothbrush and floss if the teeth are crowded or overlapped. This condition therefore may contribute to the problem of dental caries.
PRESENCE OF DENTAL APPLIANCES AND RESTORATIONS
Rosenbloom and Tinanoff evaluated the S. mutans level of patients before, during, and after orthodontic treatment.43 S. mutans levels were significantly elevated during active treatment. When samples were taken 6 to 15 weeks into the retention phase of treatment, however, the microbial levels were found to have decreased significantly to levels comparable to those of untreated children.
Dentists have known for many years that the tooth structure at the interface with restorative material is especially vulnerable to recurrent caries. Clinical studies suggest that dentists and their patients should not expect successful restorative treatment to reduce a patient’s risk for future development of carious lesions. Tinanoff and colleagues found higher numbers of salivary S. mutans in patients after they received restorative treatment.44 Wright and colleagues observed significant reductions in the number of mutans streptococci and lactobacilli immediately after restoration; however, mutans streptococci returned to prerestoration levels in many of their subjects.45 Gregory and colleagues observed that postoperative counts of salivary streptococci essentially equaled the preoperative counts after all restorative work had been completed in their patients.46 Effective prevention programs are required to protect the patient from additional caries and to better justify the investment in restorative care.
HEREDITARY FACTORS
Although parents of children with excessive or rampant caries tend to blame the condition on hereditary factors or tendencies, and some scientific evidence, as reviewed in Chapter 6, acknowledges certain genetic influences on the caries process, most authors agree that genetic influences on dental caries are relatively minor in comparison with the overall effect of environmental factors. The fact that children acquire their dietary habits, oral hygiene habits, and oral microflora from their parents makes dental caries more an environmental than a hereditary disease. Although several hereditary factors identified in Chapter 6 may be influential in promoting or preventing dental caries activity, available effective preventive therapies along with proper dietary and plaque control measures can override the hereditary factors that contribute to caries development.
EARLY DETECTION OF DISEASE ACTIVITY
Tactile probing procedures are no longer used for caries detection in most European countries and this protocol has now been adopted by many U.S. dental schools. The primary concerns that led to the discontinuation of the probing procedure were (1) the insertion of the probe into the suspected lesion inevitably disrupts the surface layer covering very early lesions, thereby eliminating the possibility for remineralizing the decalcified area; (2) the probing of lesions and suspected lesions results in the transport of cariogenic bacteria from one area to another; and (3) frank lesions requiring restoration are generally apparent visually without the need for probing. The clinical caries detection procedures commonly used in Europe have been described by several clinical investigators.47–51
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses