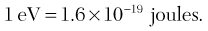The production, properties and interactions of X-rays
Introduction
X-rays and their ability to penetrate human tissues were discovered by Röentgen in 1895. He called them X-rays because their nature was then unknown. They are in fact a form of high-energy electromagnetic radiation and are part of the electromagnetic spectrum, which also includes low-energy radiowaves, television and visible light (see Table 2.1).
Table 2.1
| Radiation | Wavelength | Photon energy |
| Radio, television and radar waves | 3 × 104 m to 100 µm | 4.1 × 10−11 eV to 1.2 × 10−2 eV |
| Infra-red | 100 µm to 700 nm | 1.2 × 10−2 eV to 1.8 eV |
| Visible light | 700 nm to 400 nm | 1.8 eV to 3.1 eV |
| Ultra-violet | 400 nm to 10 nm | 3.1 eV to 124 eV |
| X-and gamma-rays | 10 nm to 0.01 pm | 124 eV to 124 MeV |
Atomic structure
Atoms are the basic building blocks of matter. They consist of minute particles – the so-called fundamental or elementary particles – held together by electric and nuclear forces. They consist of a central dense nucleus made up of nuclear particles – protons and neutrons – surrounded by electrons in specific orbits or shells (see Fig. 2.1).
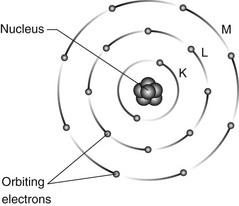
Fig. 2.1 Diagrammatic representation of atomic structure showing the central nucleus and orbiting electrons.
Useful definitions
• Atomic number (Z) – The number of protons in the nucleus of an atom
• Neutron number (N) – The number of neutrons in the nucleus of an atom
• Atomic mass number (A) – Sum of the number of protons and number of neutrons in an atom (A = Z + N)
• Isotopes – Atoms with the same atomic number (Z) but with different atomic mass numbers (A) and hence different numbers of neutrons (N)
• Radioisotopes – Isotopes with unstable nuclei which undergo radioactive disintegration.
Main features of the atomic particles
• Mass = 1/1840 of the mass of a proton
• Charge = negative: −1.6 × 10−19 coulombs
• Electrons move in predetermined circular or elliptical shells or orbits around the nucleus
• The shells represent different energy levels and are labelled K,L,M,N,O outwards from the nucleus
• The shells can contain up to a maximum number of electrons per shell:
• Electrons can move from shell to shell but cannot exist between shells – an area known as the forbidden zone
• To remove an electron from the atom, additional energy is required to overcome the binding energy of attraction which keeps the electrons in their shells.
Summary of important points on atomic structure
• In the neutral atom, the number of orbiting electrons is equal to the number of protons in the nucleus. Since the number of electrons determines the chemical behaviour of an atom, the atomic number (Z) also determines this chemical behaviour. Each element has different chemical properties and thus each element has a different atomic number. These form the basis of the periodic table.
• Atoms in the ground state are electrically neutral because the number of positive charges (protons) is balanced by the number of negative charges (electrons).
• If an electron is removed, the atom is no longer neutral, but becomes positively charged and is referred to as a positive ion. The process of removing an electron from an atom is called ionization.
• If an electron is displaced from an inner shell to an outer shell (i.e. to a higher energy level), the atom remains neutral but is in an excited state. This process is called excitation.
• The unit of energy in the atomic system is the electron volt (eV):
X-ray production
X-rays are produced inside machines, so called X-ray generating equipment, which are described in more detail in Chapter 3. However, a typical dental X-ray machine is shown in Fig. 2.2A. The X-ray generating part is referred to as the tubehead (see Fig. 2.2B), within which is a small evacuated glass envelope called the X-ray tube (see Fig. 2.2C and D). X-rays are produced inside the X-ray tube when energetic (high speed) electrons bombard the target and are suddenly brought to rest.
Main features and requirements of an X-ray tube
• The cathode (negative) consists of a heated filament of tungsten that provides the source of electrons.
• The anode (positive) consists of a target (a small piece of tungsten) set into the angled face of a large copper block to allow efficient removal of heat.
• A focusing device aims the stream of electrons at the focal spot on the target.
• A high-voltage (kilovoltage, kV) connected between the cathode and anode accelerates the electrons from the negative filament to the positive target. This is sometimes referred to as kVp or kilovoltage peak, as explained later in Chapter 3.
• A current (milliamperage, mA) flows from the cathode to the anode. This is a measure of the quantity of electrons being accelerated.
• A surrounding lead casing absorbs unwanted X-rays as a radiation protection measure since X-rays are emitted in all directions.
Practical considerations
The production of X-rays can be summarized as the following sequence of events:
1. The filament is electrically heated and a cloud of electrons is produced around the filament.
2. The high-voltage (potential difference) across the tube accelerates the electrons at very high speed towards the anode.
3. The focusing device aims the electron stream at the focal spot on the target.
4. The electrons bombard the target and are brought suddenly to rest.
5. The energy lost by the electrons is transferred into either heat (about 99%) or X-rays (about 1%).
6. The heat produced is removed and dissipated by the copper block and the surrounding oil.
7. The X-rays are emitted in all directions from the target. Those emitted through the small window in the lead casing constitute the beam used for diagnostic purposes.
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses