Properties of Materials
Objectives
After reading this chapter, the student should be able to:
< ?mpslid E1?>< ?mpslid S2?>
< ?mpslid E2?>< ?mpslid S3?>
< ?mpslid E3?>< ?mpslid S4?>
4. Describe when wettability of tooth structure or dental materials is important clinically.
< ?mpslid E4?>< ?mpslid S5?>
5. Define stress and strain, and illustrate how they differ.
< ?mpslid E5?>< ?mpslid S6?>
< ?mpslid E6?>< ?mpslid S7?>
< ?mpslid E7?>< ?mpslid S8?>
8. Describe how resilience and toughness differ from strength properties.
< ?mpslid E8?>< ?mpslid S9?>
< ?mpslid E9?>< ?mpslid S10?>
< ?mpslid E10?>
Key Terms
Coefficient of thermal expansion
Compressive strength
Corrosion
Dimensional change
Ductility
Elastic modulus
Forces: compressive, tensile, shear, twisting moment, bending moment (flexure)
Galvanism
Hydrophilic
Hydrophobic
Knoop hardness
Malleability
Newton
Percent elongation
Percolation
Proportional limit
Resilience
Shear strength
Sorption
Strain
Stress
Tarnish
Tensile strength
Thermal conductivity
Toughness
Ultimate strength
Wettability
Yield strength
An understanding of the physical, electrical, and mechanical properties of materials used in dentistry is of tremendous importance. First, materials used to replace missing portions of teeth are exposed to attack by the oral environment and subjected to biting forces. Second, the restorative materials are cleansed and polished by various prophylactic procedures. As a result, their properties are the basis for the selection of materials to be used in particular dental procedures and restorations. Clinical experience and research have related clinical success to certain properties of materials, which have been used as guides in the improvement of dental materials. Third, the establishment of critical physical properties for various types of dental materials has led to the development of minimum standards, or specifications. The American National Standards Institute (ANSI) and the American Dental Association (ADA), in conjunction with the International Organization for Standardization (ISO) and federal organizations, have established more than 100 standards, or specifications, for dental materials and maintain lists of materials that satisfy the minimum standards of quality. This information is available from the ADA office in Chicago or on its website (www.ada.org) and is helpful for selecting materials for dental practice and ensuring the quality control of materials.
This chapter emphasizes the dimensional change, electrical properties, solubility and sorption, and mechanical properties of dental materials. Selection of materials should be influenced by their effect on the oral tissues and by possible toxic effects if ingested. The color and optical qualities of materials also are important in the selection of restorative materials.
Dimensional Change
Maintaining dimensions during dental procedures such as preparing impressions and models is important in the accuracy of dental restorations. Dimensional changes may occur during setting as a result of a chemical reaction, such as with elastomeric impression materials or resin composite restorative materials or from the cooling of wax patterns or gold restorations during fabrication. To compare materials easily, the dimensional change usually is expressed as a percentage of an original length or volume (see an example calculation in Appendix 2-1). Values for other elastomeric impression materials can be used to compare their accuracy.
Volumetric dimensional change is more difficult to measure and is not described here. The volumetric dimensional change is equal to three times the linear dimensional change for a specific material.
Thermal Dimensional Change
Restorative dental materials are subjected to temperature changes in the mouth. These changes result in dimensional changes in the materials and to the neighboring tooth structure. Because the thermal expansion of the restorative material usually does not match that of the tooth structure, a differential expansion occurs that may result in leakage of oral fluids between the restoration and the tooth.
The linear thermal expansion of materials can be measured by determination of the difference in length of a specimen at two temperatures (see an example calculation in Appendix 2-1). To make a comparison between materials easier, the linear thermal expansion is expressed as a coefficient of thermal expansion. Typical values for selected restorative dental materials and human teeth are listed in Table 2-1.
TABLE 2-1
| MATERIAL | COEFFICIENT (× 106/°C) |
| Human teeth | 8–15 |
| Ceramics | 8–14 |
| Glass ionomer base | 10–11 |
| Gold alloys | 12–15 |
| Dental amalgam | 22–28 |
| Composites | 25–68 |
| Unfilled acrylics and sealants | 70–100 |
| Inlay wax | 300–1000 |
The thermal coefficient of expansion is not uniform throughout the entire temperature range and is usually higher for liquids than for solids. The thermal coefficient of expansion for a solid, such as a dental wax, generally increases at some point as the temperature is increased. The linear rather than the volumetric coefficient of thermal expansion usually is reported.
The relationship between the coefficients of thermal expansion of human teeth and restorative materials is important, and Table 2-1 shows that the values for amalgam and composites are about three to five times those of human teeth. The values for unfilled polymers, however, are five to seven times those of teeth, with ceramic being ½ to ⅓ and gold alloys being approximately the same as for human teeth.
A clinical effect of this difference is as follows. If a tooth contained a poorly bonded composite restoration that was cooled by the drinking of a cold liquid, the restoration would contract more than the tooth, and small gaps would result at the junction between the two materials. Oral fluids can penetrate this space. When the temperature returns to normal, this fluid is forced out of the space. This phenomenon is called percolation and occurs with some restorative materials, depending on the relationship of the thermal coefficient of expansion of the material and human teeth and the extent of bonding. Percolation is thought to be undesirable because of the possible irritation to the dental pulp and recurrent decay. Dental amalgam is unusual in that percolation decreases with time after insertion, presumably as a result of the space being filled with corrosion products from the amalgam. If the aforementioned composite were bonded adequately to the tooth, the difference in thermal coefficient of expansion could result in stress at the interface, which could lead to failure of the bond over time.
Thermal Conductivity
Qualitatively, materials have different rates of conducting heat; metals have higher values than polymers and ceramics. When a portion of a tooth is replaced by a metal restoration such as amalgam or gold alloy, the tooth may be temporarily sensitive to temperature changes in the mouth. Individuals who wear orthodontic appliances or complete acrylic dentures also notice temperature effects different from those experienced without these appliances.
Thermal conductivity has been used as a measure of the heat transferred and is related to the rate of heat flow (see more details in Appendix 2-1). The thermal conductivity of a variety of materials is reported in Table 2-2.
TABLE 2-2
Thermal Conductivity of Dental Materials
| Material | THERMAL CONDUCTIVITY (cal/sec/cm2[°C/cm]) |
| Unfilled acrylics | 0.0005 |
| Zinc oxide–eugenol cement | 0.0011 |
| Human dentin | 0.0015 |
| Human enamel | 0.0022 |
| Composites | 0.0025 |
| Ceramic | 0.0025 |
| Zinc phosphate cement | 0.0028 |
| Dental amalgam | 0.055 |
| Gold alloys | 0.710 |
Human enamel and dentin are poor thermal conductors compared with gold alloys and dental amalgam, although amalgam is substantially lower than gold. Glass ionomer cement bases closely replace lost tooth structure with respect to thermal conductivity. The reason for using cements as thermal insulating bases in deep cavity preparations is that although dentin is a poor thermal conductor, a thin layer of it does not provide enough thermal insulation for the pulp unless a cement base is used under the metal restoration. Composite restorations have thermal conductivities comparable to tooth structure and do not present a problem with this property. Cavity varnishes and liners have low thermal conductivities but are used in layers so thin that they are ineffective as thermal insulators.
Electrical Properties
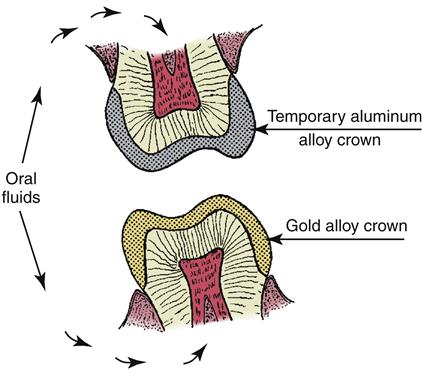
Two electrical properties of interest are galvanism and corrosion. Galvanism results from the presence of dissimilar metals in the mouth. Metals placed in an electrolyte (a liquid that contains ions) have various tendencies to go into solution. Aluminum, alloys of which are sometimes used as temporary crowns, has a strong tendency to go into solution and has an electrode potential of +1.33 volts. Gold, on the other hand, has little tendency to go into solution, as indicated by an electrode potential of −1.36 volts. A schematic sketch of two opposing teeth, one with a temporary aluminum alloy crown and the other with a gold crown, is shown in Figure 2-1. The oral fluids function as the electrolyte, and the system is similar to that of an electrical cell. When the two restorations touch, current flows because the potential difference is 2.69 volts, and the patient experiences pain and frequently complains of a metallic taste. The same effect can be experienced if some aluminum foil from a baked potato becomes wedged between two teeth and contacts a gold restoration. Temporary polymer crowns are used to prevent this problem because they are poor electrical conductors.
Corrosion also can result from this same condition when adjacent restorations are of dissimilar metals. As a result of the galvanic action, material goes into solution, and roughness and pitting occur. This effect also may occur if a gold alloy is contaminated with a metal such as iron during handling in the dental laboratory or because of variations in concentration of elements from one part of the restoration to another. Corrosion also may result from chemical attack of metals by components in food or saliva. Dental amalgam, for example, reacts with sulfides and chlorides in the mouth, as shown by polished amalgams becoming dull and discolored with time. This effect sometimes is referred to as tarnish.
Solubility and Sorption
The solubility of materials in the mouth and the sorption (adsorption plus absorption) of oral fluids by the material are important criteria in their selection. Frequently, laboratory studies have evaluated materials in distilled water. At times, these studies gave results that were inconsistent with clinical observations, because materials in the mouth are covered with plaque and therefore are exposed to various acids and organic materials. An example of the inconsistency is that zinc phosphate cements are considerably more soluble in the mouth than in laboratory tests in water indicate. Also, the loss of zinc phosphate cement retaining a gold crown is a result of dissolution followed by and accompanied by disintegration. Nevertheless, laboratory tests usually rank materials correctly, so only the actual magnitude of the numbers should be taken with a grain of salt.
Solubility and sorption are reported in two ways: (1) in weight percentage of soluble or sorbed material and (2) as the weight of dissolved or sorbed material per unit of surface area (e.g., milligrams per cm2).
Absorption refers to the uptake of liquid by the bulk solid; for example, the equilibrium absorption of water by acrylic polymers is in the range of 2%. Adsorption indicates the concentration of molecules at the surface of a solid or liquid, an example being the adsorption of components of saliva at the surface of tooth structure or of a detergent adsorbed on the surface of a wax pattern.
Wettability
The wettability of solids by liquids is important in dentistry; for example, the wetting of denture base acrylics by saliva, the wetting of tooth enamel by pit and fissure sealants, the wetting of elastomeric impressions by water mixes of gypsum materials, and the wetting of wax patterns by dental investments.
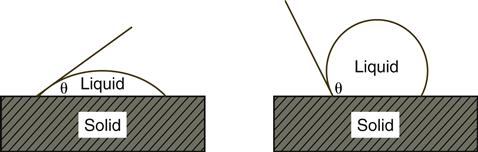
The wettability of a solid by a liquid can be observed by the shape of a drop of the liquid on the solid surface. Profiles of drops of liquids on solids are shown in Figure 2-2. The shape of the drops is identified by the contact angle θ, by the angles through the drops bounded by the solid surface, and by a line through the periphery of the drop and tangent to the surface of the liquid.
If a low contact angle occurs, as in the left of Figure 2-2, the solid is wetted readily by the liquid (hydrophilic if the liquid is water). If a contact angle is greater than 90°, as in the right of Figure 2-2, poor wetting occurs (hydrophobic if the liquid is water).
The degree of wetting depends on the relative surface energies of the solids and the liquids and on their intermolecular attraction. High-energy solids and low-energy liquids encourage good wetting; thus, liquids generally wet higher-energy solids well (e.g., water on metals and oxides). On the other hand, liquids bead up on lower-energy solids such as wax, Teflon, and many polymers. The high contact angle of water on these solids can be decreased by adding a wetting agent such as a detergent to the water, thus lowering the surface tension or energy.
Mechanical Properties
Knowledge of the magnitude of biting forces is essential in understanding the importance of the mechanical properties of dental materials. Maximum biting forces decrease from the molar to the incisor region, and the average biting forces on the first and second molars are about 580 Newtons (N), whereas the average forces on bicuspids, cuspids, and incisors are about 310, 220, and 180 N, respectively. To convert Newtons to pounds, Newtons are divided by 4.45.
Patients exert lower biting forces on bridges and dentures than on their normal dentition. For example, when a first molar is replaced by a fixed bridge, the biting force on the restored side is approximately 220 N compared with 580 N when the patient has natural dentition. The average biting force on partial and complete dentures has been measured to be about 111 N; therefore, patients with dentures can apply only approximately 19% of the force of those with normal dentition.
Stress
When a force is applied to a material, the material inherently resists the external force. The force is distributed over an area, and the ratio of the force to the area is called the stress (see more details in Appendix 2-1).
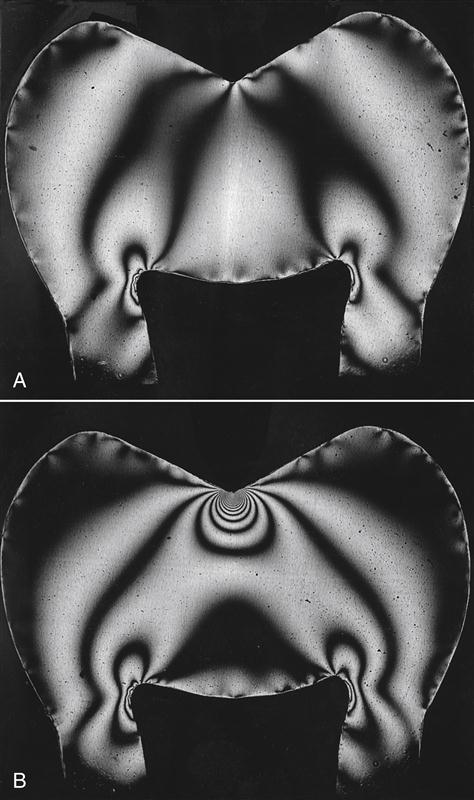
Thus, for a given force, the smaller the area over which it is applied, the larger the value of the stress. Figure 2-3 illustrates this effect. A distributed force has been applied in Figure 2-3, A, and the same force has been applied in a concentrated manner in Figure 2-3, B. The number of lines (fringes) in the plastic model of a tooth when examined in polarized light is directly proportional to the stress, and the stress is shown to be inversely proportional to the area of application. This effect can be demonstrated as follows: an unsharpened pencil is placed against the palm of the hand; a force is applied by placing a book on the end with the eraser; and any pain is noted. Then the pencil is sharpened; the procedure is repeated; and the increase in pain is noted as a result of the increase in stress.
The relationship of force, area, and stress is shown also in Table 2-3. A force of 111 N, which can readily be applied in the mouth, can produce a large stress, such as 172 megapascals (or MPa), when the area of application of the force is small. One megapascal equals approximately 145 lbs/in2. Such conditions readily exist in the mouth, where contact areas of 0.6 mm2 frequently occur.
TABLE 2-3
Relationship of Force, Area, and Stress
| FORCE (N) | AREA (mm2) | STRESS (MPa) |
| 111 | 645 | 0.1724 |
| 111 | 64.5 | 1.724 |
| 111 | 6.45 | 17.24 |
| 111 | 0.645 | 172.4 |
| 111 | 0.0645 | 1724.0 |
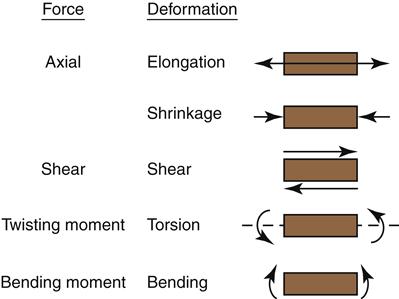
Several types of stress may result when a force is applied to a material. These forces are referred to as compressive, tensile, shear, twisting moment, and bending moment (flexure) and are shown diagrammatically in Figure 2-4. A material is subjected to compressive stress when the material is squeezed together, or compressed, and to tensile stress when pulled apart. Shear stress occurs when one portion (plane) of the material is forced to slide by another portion. These types of stresses are considered to evaluate the properties of various materials.
Strain
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses