Pharmacology
• Penicillin Allergy/Anaphylaxis
• Antibiotic-Associated Colitis
• Acute Acetaminophen Toxicity
Penicillin Allergy/Anaphylaxis
Examination
Maxillofacial. Examination is consistent with a mandibular body fracture.
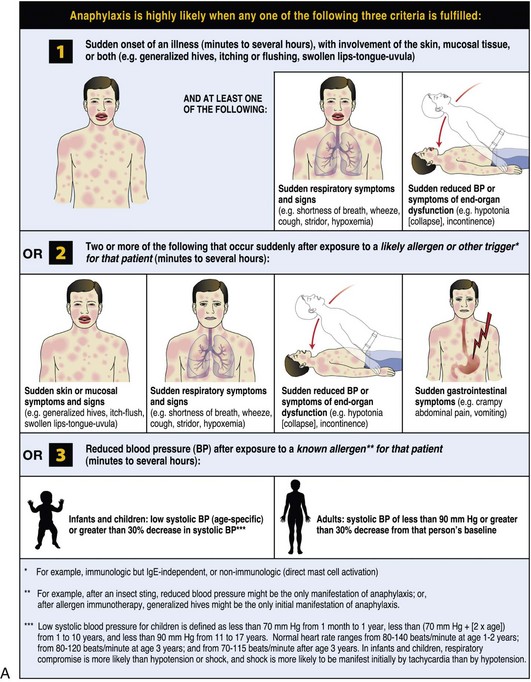
The World Allergy Organization (WAO) has developed a poster that presents the key clinical criteria for both the diagnosis and initial treatment of anaphylaxis (Figure 2-1). These criteria reflect the different clinical presentations; anaphylaxis is highly likely when any one of the criteria is met. It was acknowledged that no single set of criteria can provide 100% sensitivity and specificity, but it is believed that the WAO’s proposed criteria are likely to capture more than 95% of cases of anaphylaxis. The majority of anaphylactic reactions include skin symptoms, which are noted in more than 80% of cases. Thus at least 80% of anaphylactic reactions should be identified by criterion 1, even when the allergic status of the patient and the potential cause of the reaction might be unknown.
Assessment
Immediate allergic anaphylactic reaction induced by intravenously administered penicillin G
Box 2-1 outlines the differential diagnosis of anaphylactic shock.
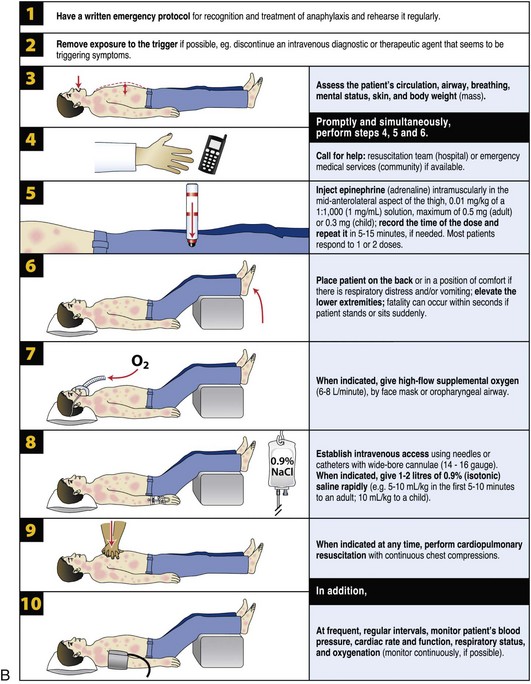
Treatment
• α1 adrenergic agonist: Increased vasoconstriction, increased peripheral vascular resistance, and decreased mucosal edema (in the upper airway)
• β1 adrenergic agonist: Increased inotropy and chronotropy
• β2 adrenergic agonist: Increased bronchodilation and decreased release of mediators from mast cells and basophils
Discussion
Most clinicians simply accept a diagnosis of penicillin allergy without obtaining a detailed history of the reaction. In their review, Salkind and colleagues stress the importance of a thorough history when faced with a penicillin-allergic patient (Box 2-2). However, it has been shown that patients with a vague history have also been found to have an IgE-mediated allergy. The time elapsed since the last reaction is important, because penicillin-specific IgE antibodies decrease with time (approximately 80% of patients with IgE-mediated penicillin allergy have lost sensitivity after 10 years). Nonetheless, it is prudent to refer any patient with a history of IgE-mediated penicillin allergy for testing. Penicillin is the most common cause of drug-induced anaphylaxis. It causes an estimated 40% to 50% of all anaphylactic deaths in the United States.
Gell, POH, Coombe, RRA. The classification of allergic mediated underlying disease. In Coombe RRA, Gell POH, eds. : Clinical aspects of immunology, ed 2, Oxford, Blackwell Science, 1968.
Gomez, MB, Torres, MJ, Mayorga, C, et al. Immediate allergic reactions to beta lactams: facts and controversies. Curr Opin Allergy Clin Immunol. 2004; 4:261.
Gruchalla, R. Drug allergy. J Allergy Clin Immunol. 2003; 111:548.
Mertes, PM, Tajima, K, Regnier-Kimmoun, MA, et al. Perioperative anaphylaxis. Med Clin North Am. 2010; 94:761.
Miller, EL. The penicillins: a review and update. J Midwifery Womens Health. 2002; 47:426.
Park, MA, Li, JT. Diagnosis and management of penicillin allergy. Mayo Clin Proc. 2005; 80:405.
Riedl, M, Casalias, A. Adverse drug reactions: types and treatment options. Am Fam Physician. 2003; 68:1781.
Romano, A, Blanca, M, Torres, MJ, et al. Diagnosis of nonimmediate reactions to β-lactam antibiotics. Allergy. 2004; 59:1153.
Salkind, AR, Cuddy, PG, Foxworth, JW. The rational clinical examin-ation—is this patient allergic to penicillin?: an evidence- based analysis of the likelihood of penicillin allergy. JAMA. 2001; 285:2498.
Sampson, H, Muñoz-Furlong, A, Campbell, RL, et al. Second symposium on the definition and management of anaphylaxis: Summary report: Second National Institute of Allergy and Infectious Disease/Food Allergy and Anaphylaxis Network Symposium. J Allergy Clin Immunol. 2006; 117(2):391.
Simons, F, Estelle, R, Ardusso Ledit, RF, et al. World Allergy Organization guidelines for the assessment and management of anaphylaxis. World Allergy Organ J. 2011; 4(2):13–37.
Tang, AW. A practical guide to anaphylaxis. Am Fam Physician. 2003; 68:1325.
Torres, MJ, Blanca, M, Fernandez, J, et al. Diagnosis of immediate allergic reactions to beta-lactam antibiotics. Allergy. 2003; 58:961.
Wendel, GD, Stark, BJ, Jamison, RB, et al. Penicillin allergy and desensitization in serious infections during pregnancy. N Engl J Med. 1985; 312:1229.
Antibiotic-Associated Colitis
PMHX/PDHX/Medications/Allergies/SH/FH
Allergies. The patient has a penicillin allergy (history of rash).
Imaging
Because this patient is relatively stable, abdominal imaging and/or endoscopy is not indicated.
Treatment
In otherwise healthy adults, the first step is to discontinue the precipitating antibiotic and to administer fluids and electrolytes to maintain hydration. For many patients, antibiotic-associated diarrhea is a mild and self-limited illness that responds to the discontinuation of antibiotics, supportive care, and fluid and electrolyte replacement. Specific pharmacotherapy for C. difficile–associated diarrhea should be initiated once the diagnosis of C. difficile has been confirmed or in highly suggestive cases of severely ill patients (Box 2-3). The use of opiates and antidiarrheal medications has previously been discouraged; however, some studies have shown that evidence supporting this hypothesis is lacking. Additionally, antimotility agents may be beneficial in providing symptomatic relief and reducing environmental contamination with infectious stool.
Discussion
The risk factors for the development of symptomatic C. difficile–associated diarrhea are summarized in Box 2-4. The most important modifiable risk factor for the development of C. difficile infection is exposure to antimicrobial agents. Even very limited exposure, such as single-dose surgical antibiotic prophylaxis, increases a patient’s risk of both C. difficile colonization and symptomatic disease. The initiating event for C. difficile colitis is disruption of colonic flora, with subsequent colonization. Depending on host factors, a carrier state or disease results. The disruption is usually caused by broad-spectrum antibiotics. Clindamycin and broad-spectrum penicillins and cephalosporins are most commonly implicated. C. difficile colitis can occur up to 8 weeks after discontinuation of antibiotics.
Bartlett, JG. Antibiotic-associated diarrhea. N Engl J Med. 2002; 346:334.
Bartlett, JG, Perl, TM. The new Clostridium difficile: What does it mean? N Engl J Med. 2005; 353:2503–2505.
Cohen, S, Gerding, DN, Johnson, S, et al. Clinical practice guidelines for Clostridium difficile infection in adults—2010: update by the Society for Healthcare Epidemiology of America (SHEA) and the Infectious Diseases Society of America (IDSA). Infect Control Hosp Epidemiol. 2010; 31(5):431–455.
Efron, P, Mazuski, J. Clostridium difficile colitis. Surg Clin North Am. 2009; 89(2):483–500.
Koo, HL, Koo, DC, Musher, DM, et al. Antimotility agents for the treatment of Clostridium difficile diarrhea and colitis. Clin Infect Dis. 2009; 48(5):598.
Loo, VG, Poirier, L, Miller, MA, et al. A predominantly clonal multi-institutional outbreak of Clostridium difficile–associated diarrhea with high morbidity and mortality. N Engl J Med. 2005; 353(23):2442–2449.
Louie, TJ, Miller, MA, et al. Fidaxomicin versus vancomycin for Clostridium difficile toxins. N Engl J Med. 2011; 364:422.
McDonald, LC, Killgore, GE, Thompson, A, et al. An epidemic, toxin gene-variant strain of Clostridium difficile. N Engl J Med. 2005; 353(23):2433–2441.
Mylonakis, E, Ryan, E, Calderwood, S. Clostridium difficile–associated diarrhea: a review. Arch Intern Med. 2001; 161:525.
Pimental, R, Choure, A. Antibiotic associated diarrhea and Clostridium difficile. In Carey WD, ed. : Cleveland Clinic: current clinical medicine: expert consult premium edition, ed 2, St Louis: Saunders, 2010.
Savola, KL, Baron, EJ, Tompkins, LS, et al. Fecal leukocyte stain has diagnostic value for outpatients but not inpatients. J Clin Micriobiol. 2001; 39:266.
Schroeder, M. Clostridium difficile–associated diarrhea. Am Fam Physician. 2005; 71(5):921.
Thomas, RV. Nosocomial diarrhea due to Clostridium difficile. Curr Opin Infect Dis. 2004; 17:323.
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


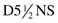 at 110 ml/hr. Clindamycin was discontinued, and the patient was started on metronidazole 500 mg orally three times daily. His diarrhea resolved in 2 days, and he was subsequently discharged. He was given a nonopiate pain medication during his hospital stay and upon discharge. The patient was educated about his diagnosis, and it was recommended that he inform other practitioners of it before initiation of antibiotic therapy.
at 110 ml/hr. Clindamycin was discontinued, and the patient was started on metronidazole 500 mg orally three times daily. His diarrhea resolved in 2 days, and he was subsequently discharged. He was given a nonopiate pain medication during his hospital stay and upon discharge. The patient was educated about his diagnosis, and it was recommended that he inform other practitioners of it before initiation of antibiotic therapy.