Oral Cancer
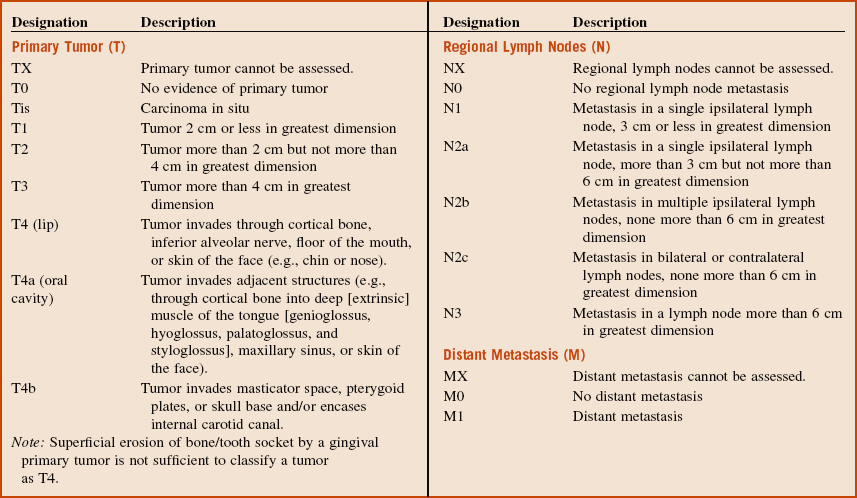
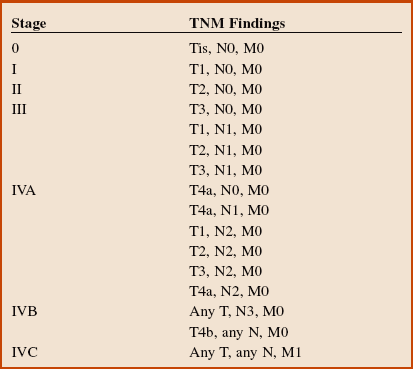
Box 11-1 outlines the TNM staging classification used for lip and oral cavity cancers; Box 11-2 looks at the staging of squamous cell carcinoma.
Squamous Cell Carcinoma*
Examination
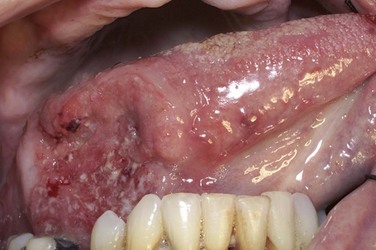
Maxillofacial. There is a 3.5-cm red and white, fungating mass on the right lateral border of tongue with central ulceration (a nonhealing ulcer in the oral cavity is considered to be SCCa until proved otherwise) (Figure 11-1). There is no pain or bleeding noted on palpation of the lesion (although ulcers from SCCa may occasionally bleed, they are usually painless). Some patients may also complain of ear pain if the lesions are deep and involve the lingual nerve. When ear pain is present, perineural invasion cannot be ruled out until final pathology. Examination of the remaining oral cavity, including the buccal mucosa, hard and soft palate, parotid and submandibular glands, oropharynx, and nasopharynx, reveals no other abnormalities. Nasopharyngoscopy reveals no abnormal tissues in the posterior oropharynx, subglottic or supraglottic regions, or nasopharynx (nasopharyngoscopy should be performed as part of the head and neck evaluation of tongue SCCa).
Treatment
A common procedure that accompanies the removal of the tumor is the removal of the fibrofatty contents of the neck, for treatment of cervical lymphatic metastases and for complete staging of the cancerous process (see the section Neck Dissections later in this chapter).
Reconstruction and rehabilitation. Depending on the defect, the reconstructive surgery can be divided into soft tissue and/or bony reconstruction. Closing the defect primarily is ideal if it can be accomplished. Soft tissue surgical procedures include closure by secondary intention, skin grafts, local flaps, or microvascular free flaps. Simultaneous bony reconstruction can be accomplished using vascularized free flaps from the iliac crest, scapula, or fibula when needed. When large ablative and reconstructive procedures are performed, they can be performed simultaneously (see Chapter 12). Depending on the amount of healing and dysfunction anticipated, a percutaneous endoscopic gastrostomy tube and elective tracheostomy can be performed to secure the airway and aid in the nutritional support of the patient during the postoperative period.
Discussion
1. Carcinomas arising in the oral cavity, which includes the tongue, gingiva, floor of the mouth, hard palate, buccal mucosa, and retromolar area
2. Carcinomas of the lip vermilion
3. Carcinomas of the oropharynx, including the base of the tongue, lingual tonsil, soft palate, and uvula
Figure 11-2 shows a different patient with a large fungating SCCa of the right retromolar area.
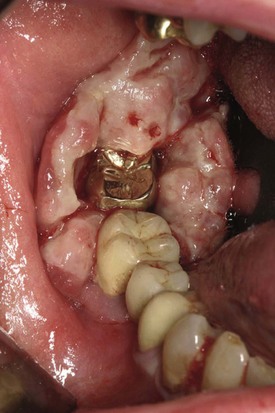
Figure 11-2 Squamous cell carcinoma of the right retromolar area in a different patient from the one shown in Figure 11-1.
Leukoplakia is a white patch or plaque that cannot be characterized clinically or pathologically as any other disease. Erythroplakia is defined as a red lesion of the oral cavity that cannot be classified clinically or pathologically as any other lesion (see the section on Oral Leukoplakia in Chapter 7).
Grade I: More than 75% differentiated cells
Grade II: 25% to 75% differentiated cells
The 5-year survival rate remains about 50% and is related to the stage at diagnosis (Table 11-1). Unfortunately, there has been only a modest improvement in survival over the past several decades.
Table 11-1
Oral Squamous Cell Carcinoma Survival Rates by Stage
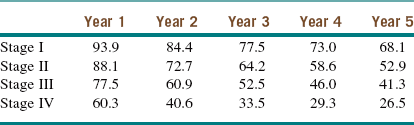
Modified from the National Cancer Institute, US National Institutes of Health: Surveillance epidemiology and end results (SEER). Available at http://seer.cancer.gov/publicdata/access.html. Accessed December 2012.
Braakhuis, BJM, Tabor, MP, Kummer, JA, et al. A genetic explanation of Slaughter’s concept of field cancerization: evidence and clinical implications. Cancer Res. 2003; 63:1727–1730.
Broders, AC. Carcinomas of the mouth: types and degrees of malignancy. Am J Roentgenol Radium Ther Nucl Med. 1927; 17:90–93.
Funk, FF, Karnell, LH, Robinson, RA, et al. Presentation, treatment, and outcome of oral cavity cancer: a national cancer data base report. Head Neck. 2002; 24:165–180.
Hillbertz, NS, Hirsch, JM, Jalouli, J, et al. Viral and molecular aspects of oral cancer. Anticancer Res. 2012; 32:4201–4212.
Kademani, D, Bell, RB, Bagheri, SC, et al. Prognostic factors for intraoral squamous cell carcinoma: the influence of histologic grade. J Oral Maxillofac Surg. 2005; 63:1599–1605.
Marur, S, D’Souza, G, Westra, WH, et al. HPV-associated head and neck cancer: a virus-related cancer epidemic. Lancet Oncol. 2010; 11:781–789.
McClure, SA, Mohaved, R, Salama, A, et al. Maxillofacial metastases: a retrospective review of one institution’s 15-year experience. J Oral Maxillofac Surg. 2012; 16(2):181–188.
Neville, BW, Day, TA. Oral cancer and precancerous lesions. CA Cancer J Clin. 2002; 52:195–215.
Oliver, AJ, Helfrick, JF, Gard, D. Primary oral squamous cell carcinoma. J Oral Maxillofac Surg. 1996; 54:949–954.
Reichart, PA, Philipsen, HP. Oral erythroplakia: a review. Oral Oncol. 2005; 41:551–561.
Schmidt, BL, Dierks, EJ, Homer, L, et al. Tobacco smoking history and presentation of oral squamous cell carcinoma. J Oral Maxillofac Surg. 2004; 62:1005–1058.
Schwartz, LH, Ozsahin, M, Zhang, GN, et al. Synchronous and metachronous head and neck carcinomas. Cancer. 1994; 74:1933–1938.
Shafer, WG, Waldron, CA. Erythroplakia of the oral cavity. Cancer. 1975; 36:1021–1028.
Smith, GI, O’Brien, CJ, Clark, J, et al. Management of the neck in patients with T1 and T2 cancer in the mouth. Br J Oral Maxillofac Surg. 2004; 42:494–500.
Syrjanen, S. Human papillomavirus (HPV) in head and neck cancer. J Clin Virol. 2005; 32S:S59–S66.
Todd, R, Donoff, RB, Wong, DTW. The molecular biology of oral carcinogenesis: toward a tumor progression model. J Oral Maxillofac Surg. 1997; 55:613–623.
Van der Meij, EHDDS, Schepman, KP, Van der Waal, I. The possible premalignant character of oral lichen planus and oral lichenoid lesions: a prospective study. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2003; 96:164–171.
Waldron, CA, Shafer, WG. Leukoplakia revisited: a clinicopathological study of 3,256 oral leukoplakias. Cancer. 1975; 36:1386–1392.
Wooglar, JA. Histological distribution of cervical lymph node metastases from intraoral/oropharyngeal squamous cell carcinomas. Br J Oral Maxillofac Surg. 1999; 37:175–180.
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses