2
General pathology
- The inflammatory response to tissue damage
- The functioning of the immune system
- The causes of cancer and different therapies to treat cancer
- The coagulation of blood and disorders which interfere with this process
- Atherosclerosis and other cardiovascular diseases
Introduction
The aim of this chapter is to introduce the reader to a broad overview and explanation of important and fundamental aspects of general pathology relevant to both dental hygienists and dental therapists. However, it is impossible to include all pathological conditions that can occur and may at some time influence clinical practice in a single chapter. A selection of texts is therefore included at the end of the chapter under ‘Further reading’, which are considered useful in directing the reader to explore additional areas of general pathology.
Inflammation and immunity
The integrity of the human body is continuously challenged by external and internal attacks. A few examples of these include:
- In the environment that surrounds us, microorganisms are the main menace.
- Physical injury can affect (oral) tissues.
- Internally we are threatened by the risk of tumour growth.
To protect our body against such injury, we possess an ingenious defence system, composed of a large number of components:
- The skin and mucosa form the first barrier of defence against intruders.
- If this barrier is passed, the intruders will be opposed by a second barrier of defence, consisting of inflammatory cells and soluble factors. Together, these two systems form the non-specific immune system, which is present at birth (innate immunity). Although this system is capable of immediately attacking the intruder, it has no specificity against it.
- When the intruder is not rapidly eliminated by the non-specific immune system, a third barrier, the specific immune system, will be mobilised. This specific immune system needs time to deploy its full effect, but has the utmost advantage in that it raises a defensive reaction specifically aimed against that intruder. In addition, after the first contact with a particular intruder, this specific defensive reaction is recorded in memory cells. As a result, a much faster and more potent reaction will result from a future confrontation with that intruder (acquired immunity). Both systems, the non-specific and the specific system, co-operate closely.
The non-specific immune system
Skin and mucosa
When intact, the skin and mucosal membranes provide the first barrier of defence. If this line is impaired, for example in a patient with severe burns, there is great risk of invasive infections with microorganisms. Often these microorganisms originate from the so-called commensal organisms. Under normal conditions, these organisms live on the skin or mucosa without causing injury. Secretory products of glands located beneath the skin and mucosa also play an important role in the defence. Examples of local defence mechanisms against potential invaders are:
- The continuous flow of saliva through the oral cavity resulting in mechanical cleansing of the oral surfaces; e.g. in the case of a dry mouth the risk of development of dental caries and oral infections is considerably increased (Figure 2.1).
- The presence of antimicrobial components, such as lysozyme and histatins, in saliva.
- Fatty acids secreted by the skin.
- The production of tears (containing lysozyme).
- The low pH in the stomach due to secretion of hydrochloric acid.
Figure 2.1 Reduced secretion of saliva has resulted in a shift in the composition of the oral flora. These patients are prone to candidiasis.
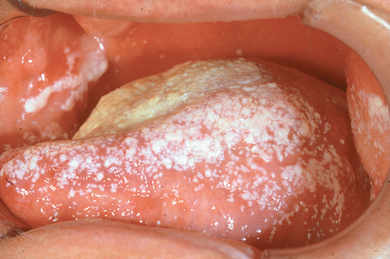
Table 2.1 Mononuclear cells.
| Location | Name | Major function (phagocytosing cells are also capable of antigen presentation) |
| Blood | Monocyte | Phagocytosis |
| Lymph node | Follicular dendritic cell | Antigen presentation |
| Thymus | Interdigitating dendritic cell | Antigen presentation |
| Brain | Microglia cell | Phagocytosis |
| Lung | Alveolar macrophage | Phagocytosis |
| Liver | Kupffer cell | Phagocytosis |
| Kidney | Mesangial cell | Phagocytosis |
| Skin | Langerhans cell | Antigen presentation |
The normal (commensal) bacterial flora of the body is also an important defence mechanism. It suppresses the growth of many (potentially) pathogenic bacteria by competition for essential nutrients or production of inhibitory substances. This so-called colonisation resistance may be disturbed by the use of antibiotics. The result may be an overgrowth of pathogenic bacteria as their natural competitors are not present in sufficient numbers any more. In the gut, for example, such a disturbance may lead to a clinical infection with diarrhoea.
Cells of the non-specific immune system
When an intruder has passed the skin and/or mucosal surfaces, it will first encounter macrophages. These cells of the non-specific immune system, differentiated from blood monocytes, are present everywhere in the body. Depending on the tissue or organ where they reside, they have a more or less specialised structure and function (Table 2.1).
The function of macrophages is twofold:
- To recognise and clear intruders.
- To degrade the intruder into small protein fragments.
First, the macrophage attaches itself to the foreign structure, often a microorganism. This structure is ‘swallowed’ due to invagination of the cellular membrane of the macrophage. In this way a phagosome is formed. This process of ‘eating’ by the cell is called phagocytosis and is significantly accelerated when the microorganism to be swallowed is covered with antibodies and/or complement. Subsequently, the phagosome fuses with lysosomes, which are membrane-surrounded cell organelles in the cytoplasm of the macrophage containing lytic enzymes and myeloperoxidase. The latter enzyme produces an oxygen-derived free radical with powerful antimicrobial activity. The combined activity of the lytic enzymes and oxygen radicals kills and destroys the foreign structure.
Second, the foreign structures are degraded into small protein fragments. These fragments, peptides with a length of 8–15 amino acids, are presented to the specific immune system. This process is a good example of the close collaboration between the non-specific and specific immune system.
If the intruder has succeeded in passing the above mentioned defence barriers and enters a blood vessel, it will be confronted with circulating phagocytosing cells (monocytes), which are comparable to the macrophages in tissues. In addition, blood contains large numbers of granulocytes. The name of these white blood cells is derived from the presence of granules in their cytoplasm. These granules contain lytic enzymes. The majority of the granulocytes are neutrophils, capable of phagocytosing foreign structures. Under certain conditions, neutrophils can leave the bloodstream and enter tissues. The eosinophils and basophils play a role in the defence against parasites.
A final group of cells from the non-specific immune system, present in the blood, are the so-called natural killer cells (NK cells). The NK cells can recognise aberrant structures on tumour cells. They make contact with these tumour cells and release the contents of their granules. The granules contain perforins, enzymes that are capable of making a hole in the membrane of the tumour cell, resulting in its death.
Table 2.2 and Figure 2.2 provide an overview of the cell types of the non-specific immune system.
Humoral factors of the non-specific immune system
Tissues and blood plasma also contain several chemical substances, mostly proteins, which are involved in the non-specific immune response. An important system is the complement system, which consists of a series of plasma proteins. Under normal conditions, these proteins are present in blood in their inactive form (Figure 2.3). The complement system can be activated in three ways:
- The classical pathway is activated by immune complexes (an antibody bound to the specific structure it recognises, e.g. part of a microorganism).
- The alternative pathway is activated by bacterial cell wall structures.
- The lectin pathway is also activated by bacterial cell wall structures.
Table 2.2 Cells of the immune system.
| Cell | Reference values (healthy individuals) | Major function |
| Non-specific immune system | ||
| Neutrophil Active eosinophil Basophil |
2–7 × 109/litre blood 0–0.4 × 109/litre blood 0–0.2 × 109/litre blood |
Phagocytosis + release of biologically active substances + release of toxic substances |
| Mast cell | Localised in tissues | Tissue pendant of basophils, involved in allergic reactions |
| Monocyte | 0.2–0.8 × 109/litre blood | Functions comparable with granulocyte + antigen presentation |
| Macrophage | Localised in tissues | Tissue pendant of monocyte |
| Dendritic cell | Localised in tissues, small percentage present in blood | Antigen presentation |
| ‘Natural killer’ cell | 0.1–1.4 × 109/litre blood | Non-specific killing of cells + release of biologically active compounds |
| Specific immune system | ||
| T-lymphocyte | 1–4 × 109/litre blood | Cellular immunity (help, suppression and cytotoxic) |
| B-lymphocyte | 0.1–1 × 109/litre blood | Humoral immunity (production of antibodies) |
Figure 2.2 Schematic illustration of the main cells of the immune system.
Figure 2.3 Simplified diagram of the complement system.
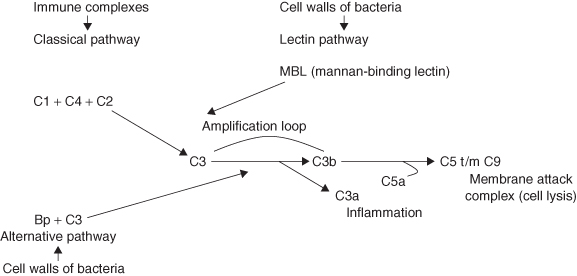
All three pathways lead to activation of the central part of the complement system, which is followed by self-amplification resulting in rapid activation of an enormous amount of complement proteins. This results in several biological effects. Some small complement fragments attract neutrophils. Other parts of the complement system form a cylinder-shaped structure in the membrane of bacteria resulting in cell lysis (destruction). Since macrophages and monocytes have receptors for complement C3b on their surface, they can phagocytose bacteria covered with this complement protein much faster.
A second important group of humoral factors is formed by the cytokines. These are low molecular weight compounds that are produced by many different cell types, including cells that are not part of the immune system. Cytokines can exert their activity on the producing cell itself (autocrine activity), on neighbouring cells (paracrine activity) or on cells far away (endocrine activity). Therefore, cytokines are the key mediators in the communication between cells.
The specific immune system
The specific immune system aims at a specific target and deploys a strong defensive reaction against that specific target. A structure that induces such a reaction is called an antigen. To be effective, this reaction needs time: approximately 7 days after the initial contact with the antigen. Simultaneously with this initial reaction an immunological memory is developed. Because of this memory, a much faster (about 2 days) and stronger reaction will occur on the next contact with the same antigen. This immunological memory forms the basis of vaccinations. First, a specific immune reaction is induced against a weakened or killed microorganism in order to raise a stronger and more rapid defensive reaction on subsequent infections with the same microorganism.
The defensive reaction itself can be exerted by antibodies and/or cells. Antibodies attach themselves to specific surface structures. This activates the classical pathway of the complement system. In addition, foreign structures covered with antibodies are phagocytosed much faster by monocytes and macrophages. Cells of tissues that have become infected with a virus or intracellular bacterium (e.g. Mycobacterium tuberculosis) will be rendered harmless by cells of the immune system.
The specific immune response consists of three phases:
- The initial phase is the so-called antigen presentation during which the antigen is presented to the specific immune system.
- During the second phase the system becomes activated and a memory against subsequent exposures with that antigen develops.
- The third phase is the effector phase. During this phase the ultimate defensive reaction takes place.
Antigen presentation
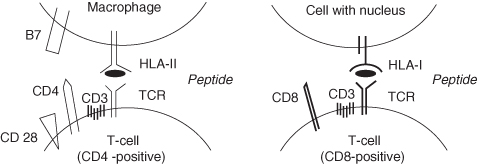
When an intruder passes the first barrier, it will be confronted with macrophages that are capable of phagocytosing the intruder and degrading it into small fragments, peptides of 8 to 15 amino acids. These peptides are admitted to the groove of human leucocyte antigen molecules (HLA molecules, see Figure 2.4). Next, the HLA molecules with peptide fragments in their groove move to the cell surface, where the peptide is presented to T-lymphocytes.
There are two classes of HLA molecules: HLA class I and HLA class II. HLA class I molecules can be subdivided into A, B and C antigens; HLA class II molecules are subdivided into DR-, DQ- and DP-antigens. HLA class I molecules are present on all cells with a nucleus; HLA class II molecules are restricted to antigen-presenting cells (macrophages, monocytes and B-lymphocytes).
Figure 2.4 HLA class I and HLA class II mediated T-cell interactions.
Each individual has its own set of HLA molecules, which differ from those of other individuals. Only monozygotic (identical) twins possess exactly the same HLA molecules. Because of these individual differences in HLA molecules, tissues or organs from another person are recognised by the immune system of the acceptor as foreign. This may result in rejection of the graft, a major problem with transplantation.
Activation of the specific immune system and development of immunological memory
The specific immune system is composed of lymphocytes. Although each lymphocyte is only capable of recognising one specific antigen, together these lymphocytes can recognise an enormous variation in foreign structures. Lymphocytes are divided into B-lymphocytes and T-lymphocytes.
B-lymphocytes develop in the bone marrow. After activation, they can start forming antibodies. B-lymphocytes carry these antibodies on their surface and use them as receptors to recognise specific antigens. The variation in B-lymphocytes, each with their own antibody, is enormous.
After being created in the bone marrow, T-lymphocytes move to the thymus to mature. There are two kinds of T-lymphocytes: those with a CD4 molecule on their surface (which mainly function as T-helper cells) and those with a CD8 molecule on their surface (which mainly function as cytotoxic cells). T-cells also have a specific receptor on their surface, the T-cell receptor (TCR). The latter receptor enables them to recognise antigenic peptides. The TCR of CD4-positive T-cells can recognise peptides presented via HLA class II molecules; the TCR of CD8-positive T-cells can recognise peptides presented via HLA class I molecules (Figure 2.4). Each T-cell has one specific TCR. As a consequence, the total variation in T-cells is, like the variation in B-lymphocytes, enormous.
When a CD4-positive T-cell meets an antigen-presenting cell with a peptide in its HLA class II molecule that exactly fits in the TCR of this T-cell, that T-cell will be activated and start to produce the cytokine interleukin 2. This interleukin acts as an autocrine growth factor for this T-cell. Thereupon this cell starts to divide. As a result, activated T-cells become available to provide help to specific B-cells to form antibodies and/or to provide help to specific cytotoxic T-cells. The T-cell exerts this action mainly by producing cytokines. Some of the created T-cells remain in the body for a long time, circulating as memory cells. These cells are promptly available during future contacts with the antigen they are raised against, and will then provide a rapid and even stronger reaction.
The effector phase of the specific immune response
By producing interleukins 4 and 10, CD4-positive T-helper cells provide assistance to B-lymphocytes. For the activation of B-lymphocytes, two signals are necessary:
- A B-cell must recognise the antigen with the antibody on its surface.
- Assistance from a specific T-cell.
If both conditions are met, the activated B-cell (plasma cell) will start to produce antibodies. After the contact with an antigen, it takes quite some time before the first antibodies are produced (the so-called primary immune response); on average 7 days. These antibodies, of the immunoglobulin M (IgM) class, bind rather weakly to the antigen. Subsequently, IgG and IgA molecules are produced which can bind much more strongly with the antigen. After a second confrontation with the antigen this process is completed much faster due to the presence of memory T-cells with specificity for the antigen. This secondary immune response produces large numbers of IgG molecules, which bind strongly to the antigen (Figure 2.5). Bacteria-covered antibodies are rapidly and efficiently removed by phagocytosing cells. The production of antibodies is especially important for the defence against encapsulated bacteria like pneumococci and Haemophilus influenzae.
Figure 2.5 The primary and secondary immune response.
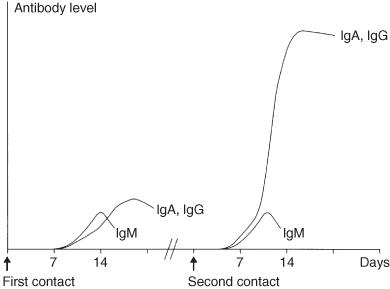
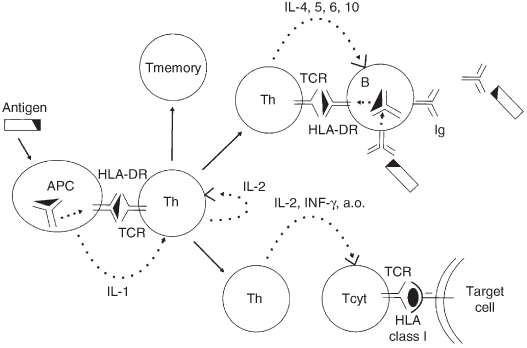
Figure 2.6 Diagram of the primary immune response. The antigen is phagocytosed by an antigen-presenting cell (APC), partially degraded and, in association with HLA class II (HLA-DR), presented to an antigen specific T-helper cell (Th) that recognises the relevant epitope with its T-cell receptor (TCR). The T-cell is activated and starts to proliferate, aided by interleukin 1 (IL-1 from the APC) and IL-2 (produced by the T-cell itself). T-memory cells and T-helper cells (Th) develop which, by direct interaction with B-cells and by production of cytokines (interleukins 4, 5 and 6), stimulate antigen specific B-cells to proliferate and to produce immunoglobulins (Ig). The T-helper cells also activate cytotoxic T-cells (Tcyt). Tcyt recognise antigen presented in association with HLA class I molecules on the target cell.
A large number of microorganisms (especially viruses, intracellular bacteria like Mycobacterium tuberculosis and certain fungi) conceal themselves from the immune system by entering the cells of the body. The infected cells, however, express on their surface HLA class I molecules containing microbial peptides. Subsequently CD8-positive T-cells are capable of recognising these strange peptides presented in an HLA class I molecule with their TCR. After recognition of the strange peptide, cytotoxic T-cells become activated and release substances like perforins that lyse the infected target cell. Specific T-helper cells provide assistance for the activation of cytotoxic T-cells by producing cytokines, mainly interleukin 2 and gamma-interferon. Cytotoxic T-cell reactions play a special role in the defence against cells infected with a virus. A detailed diagram of the primary immune response is provided in Figure 2.6.
Inflammation
Acute inflammation
The development of an acute inflammatory response is well illustrated by the cascade of reactions that follows on penetration of the oral mucosa by a bacterium. In the tissue, the bacterium will be confronted with a macrophage that tries to phagocytose it. This process is facilitated by the complement system and, if immunity against that bacterium is present, specific antibodies. The macrophage becomes activated and starts to release cytokines, such as interleukin 1 and tumour necrosis factor (TNF). These cytokines exert an effect on the adjacent endothelium (epithelial lining) of capillaries. The endothelium becomes activated, resulting in a local expression of adhesion molecules on the endothelial surface. The blood vessels increase in diameter (vasodilation), which increases the blood flow through the vessels. Since the permeability of the endothelium increases simultaneously, the transport of fluid containing complement and antibodies to the tissue will increase.
Circulating neutrophils have structures on their surfaces that recognise the adhesion molecules that are expressed on the endothelium. As a result, the neutrophils adhere to the wall of the blood vessel and begin to move in the direction of the activated macrophage. This process is guided by substances released from the macrophage, the so-called chemotactic factors. In the area where bacteria have penetrated the mucosa, granulocytes are urgently needed because bacteria usually invade in large numbers. The granulocytes also begin to phagocytose bacteria, again helped by complement and specific antibodies. In doing so, the granulocytes become activated and release their lytic enzymes. This causes tissue damage and often the granulocytes have ‘dug their own grave’ as well. The disrupted granulocytes and necrotic tissue form pus.
This inflammatory reaction induces the five characteristic local clinical signs of acute inflammation, of which the first four were first described by the Greek doctor Celsus in the first century BCE:
- Redness: due to the local dilation of small blood vessels.
- Heat: vasodilation results in an increased delivery of warm blood to the area.
- Swelling: due to accumulation of fluid in the tissue.
- Pain: pus and swelling of the tissues press on nerve endings. Some chemical mediators of inflammatory response are also able to induce pain.
In the nineteenth century, the German Rudolf Virchow added a fifth sign:
- Loss of function: movements of an inflamed area are reflex inhibited by pain. In addition severe swelling may physically immobilise the tissues.
Inflammation of a specific tissue is denoted by the suffix ‘-itis’ added to the name of the tissue. For example, an inflammatory response in the gingiva is called gingivitis, in the periodontium, periodontitis and in the mucosa, mucositis, etc.
In the case of a more extensive and/or longer-lasting local inflammatory reaction, a systemic reaction may occur that involves the other parts of the body. The cytokines interleukin 1 and 6, originating from the inflamed area, reach the brain where they reset the body’s ‘thermostat’, inducing a higher body temperature (fever). In addition, these interleukins activate the liver to produce acute phase proteins. These proteins enhance host resistance and minimise tissue injury. Acute phase proteins are also responsible for the increase in the sedimentation rate of erythrocytes during inflammation, known as the erythrocyte sedimentation rate (ESR), which is often used as a clinical parameter of disease activity.
Chronic inflammation
When the inflammatory agent persists, the character of the inflammatory response changes. This can, for example, be due to extremely virulent bacteria that are resistant to breakdown by the acute inflammatory response, or through the inability of a deficient immune system to clear microbes. In chronic inflammation, neutrophils become replaced by macrophages. Since the infectious agent persists, the immune response also plays a more prominent role. Therefore, a chronic infiltrate is composed of macrophages, lymphocytes and plasma cells.
A specific form of chronic inflammation is granulomatous inflammation. This type of chronic inflammation is characterised by the development of granulomas: large accumulations of macrophages and lymphocytes with a diameter of several millimetres. Granulomas also contain modified macrophages, which are called epithelioid cells because they resemble epithelial cells. Fusion of several epithelioid cells may result in multinucleated giant cells (Langerhans’ cells), which possess some phagocytic activity. This type of chronic inflammation is especially observed in the reaction to infection with intracellular living pathogens, such as M. tuberculosis, which is resistant to digestion after phagocytosis. It can also be observed in the reaction to tissue damage by foreign non-digestible substances, such as silica particles and suture materials, or infections with Actinomyces species. The latter microorganisms are commensals of the oral cavity and only cause an inflammatory reaction in special cases, often related to trauma or a surgical procedure (e.g. local anaesthesia or the extraction of teeth).
Chronic inflammation is characterized by a sustained inflammatory process whose duration may vary from several months to years. In many cases chronic inflammation does not induce complaints and patients are often unaware of the chronic inflammation. For a definitive treatment, the cause of the chronic inflammation should be removed. This phenomenon is nicely illustrated by treatment of periodontitis as removal of accumulated plaque is an important element in the treatment of chronic periodontitis.
During the last decades, several studies showed a relation between periodontitis and systemic diseases, such as cardiovascular diseases, pulmonary diseases, diabetes mellitus and rheumatoid arthritis. This relationship will be described in more detail in Chapter 5, p. 99.
Wound healing
Primary intention
When the noxious agent is killed and removed, the body will start to repair the damage. An injury with little loss of tissue, for example a surgical incision, will be healed by primary intention (the healing together of clean, closely opposed wound edges). Since blood vessels are cut, some bleeding occurs, followed by aggregation of blood platelets and the production of fibrin. Together with clumped red blood cells, they form a clot that covers the wound and prevents infection. In fact, an acute inflammatory reaction is observed in the area of repair. Macrophages release cytokines that activate fibroblasts, myofibroblasts and endothelial cells residing in the wound margins. Guided by the meshwork of fibrin, these cells begin to grow into the clot, forming granulation tissue. This tissue is gradually replaced by collagen fibrils. The final result is a thin white line of collagen visible under the transparent layer of regenerated epithelium: a />
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


