Endodontic materials
Introduction
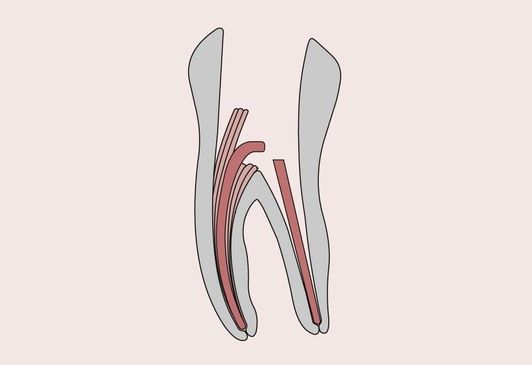
Endodontics is concerned with the morphology, physiology and pathology of the human dental pulp and periradicular tissues. Endodontic treatment is aimed at saving the tooth when injury to the pulp and associated periradicular tissues has occurred. Treatments involving the use of dental materials include capping of an exposed vital pulp, sealing of the root canal space when the pulp has had to be removed and, in the case of badly broken down teeth, reconstruction with endodontic post and core systems (Figure 2.6.1).
Vital pulp capping
Two main causes of pulpal exposure are:
In each of the above instances, remedial treatment is necessary to save the tooth. The nature of this treatment depends on which of the above causes of pulpal injury applies.
Indirect pulp capping
Sir John Tomes stated in 1859 that ‘It is better that a layer of discolored dentine be allowed to remain for the protection of the pulp rather than run the risk of sacrificing the tooth.’ He had observed that discoloured and demineralized dentine could be left behind in deep cavities of the tooth before restoration, often with highly satisfactory results. This is especially applicable if micro-exposures of the pulp are suspected. The removal of this dentine may lead to exposure of the pulp, thus impairing its prognosis. It has been shown that demineralized dentine, if it is free of bacteria, will remineralize once the source of the infection has been eliminated. The diagnosis of the presence of demineralized dentine that is caries-free can be assisted by using a caries-disclosing solution. The placement of a suitable material directly on this demineralized dentine is commonly called indirect pulp capping (IPC) and it has to be said that there is as yet no clear consensus for the acceptance of this clinical procedure.
IPC has been defined as the steps undertaken to protect a vital tooth where removal of all affected tissues would result in a pulpal exposure. In this context, a non-exposed pulp is one that exhibits no signs of haemorrhage at or near the pulp chamber. When carrying out such a procedure, it is vitally important that the infection is removed and is not allowed to recur. This can be achieved with the placement of an antibacterial liner such as calcium hydroxide or zinc oxide–eugenol cement, which is aimed at stimulating secondary dentine formation. Of course, resin composites should not be placed directly on eugenol-based liners because they interfere with the polymerization of the resin.
With the advent of adhesive dental materials, another possible restorative option is the placement of calcium hydroxide cement, followed by an adhesive liner such as glass–ionomer (GIC) or resin-modified glass–ionomer cement (RMGIC). Yet another option is the use of resin composites in conjunction with a dentine-bonding agent. The aim is to provide a combination of an antibacterial barrier and an adhesive seal against the further ingress of bacteria, thus removing the pulpal antagonist and allowing the pulp to heal. When calcium hydroxide is used in this way, it should be applied sparingly so as to ensure that as much dentine is available for further bonding with the GIC/RMGIC or dentine-bonded resin composite.
More recently, an adhesive approach involving the direct application of a dentine-bonding agent has been proposed. This is aimed at sealing the remaining dentine by the creation of a hybrid zone, thus preventing immediate postoperative sensitivity and protecting the tooth from the ingress of bacteria. There are those who have serious reservations about acid etching dentine so close to the pulp, but as Figure 2.6.2 shows, the demineralization of the dentine by the penetration of acid is only a matter of a few micrometres. Further evidence exists to support the position that acid etching of dentine will not kill the underlying pulp. With regard to the stimulation of secondary dentine, more evidence has been gathered to suggest that this is not a feature unique to calcium hydroxide cement.
Direct pulp capping
Direct pulp capping can be described as the dressing of an exposed pulp with the objective of maintaining pulp vitality. If a pulpal exposure occurs as a consequence of tooth preparation or trauma, it is important that steps are taken to avoid bacterial contamination. If one can be sure that this is the case, then the procedure of direct pulp capping carries a good prognosis for saving the pulp; although views vary, the general consensus is that calcium hydroxide cement is the choice of pulp-capping material in such situations. However, this view is now being challenged, with some clinicians promoting the merits of mineral trioxide aggregate.
Nevertheless, many clinicians believe that the long-term success rate with root-canal therapy does not warrant the treatment of traumatic pulp exposures with the more unpredictable pulp-capping procedures, especially as unsuccessful pulp capping may lead to resorption, calcification, pulpitis, pulp necrosis or periapical involvement. However, the advantages of a successful pulp-capping procedure are that young vital teeth have an opportunity to continue to develop and the tooth-weakening effects of root-canal treatment are avoided.
If direct pulp capping as a clinical procedure is controversial, then so is the choice of materials for pulp capping. A pulp-capping material can be considered to behave as a wound dressing for the exposed pulp. Such a material can passively wall off the pulp from the outside environment so as to prevent bacterial invasion and/or can induce some change in the pulp.
There is evidence to suggest that the pulp has the capacity to wall itself off by forming a connective tissue barrier that eventually changes into hard tissue. The induction of hard tissue formation needs to be preceded by a low-grade irritation that results in superficial coagulation necrosis. On this basis, a pulp-capping material must:
In other words, a pulp-capping material needs to be able to interact with the pulp to initiate hard tissue formation and, once this process has been triggered, should adopt a passive role.
If the pulp is exposed due to the presence of caries, the procedure for pulp capping is contraindicated; the infiltration of bacteria that will have occurred into the pulp cannot be reversed, and the only solution is a full pulpectomy.
Pulp-capping materials
Until recently, the only material that appeared to satisfy the requirements for pulp capping was calcium hydroxide cement, which was first used for this purpose in the 1930s. However, as already mentioned, the dominant position of calcium hydroxide as the preferred pulp-capping material is now being challenged by MTA and, to some degree, by the dentine-bonding agents.
Calcium hydroxide cements
The first use of Ca(OH)2 was in the form of a slurry, consisting of no more than a mixture of calcium hydroxide in water. This was changed to a paste using methyl cellulose, being somewhat easier to handle. In the early 1960s, the hard-setting calcium hydroxide cements were introduced, in which the calcium hydroxide reacts with a salicylate ester chelating agent in the presence of a toluene sulphonamide plasticizer (see Chapter 2.4). The hard-setting cements are either two-paste systems or are single-paste systems consisting of calcium hydroxide-filled dimethacrylates, polymerized by light.
The problem with the non-setting versions is that these will gradually dissolve and disappear from underneath the restoration, which can undermine the restoration’s function. The hard-setting versions are therefore generally preferred, as these are less soluble. The difficulty for the manufacturer is to achieve a balance between a material that is sufficiently soluble to be therapeutic and not so soluble as to dissolve away, although whether the pulp-capping material needs to release anything to stimulate dentine bridge formation is arguable.
When the paste is brought in contact with the pulp it causes a layer of necrosis some 1.0–1.5 mm thick, which eventually develops into a calcified layer. Experiments using radioactive calcium in the paste have shown that the calcium salts necessary for mineralization of the bridge are not derived from the cement, but are instead supplied by the tissue fluids of the pulp. Once the bridge has become dentine-like in appearance and the pulp has been shut off from the source of the irritation, the hard tissue formation ceases. It is believed that the high pH of the calcium hydroxide cement is responsible for this type of pulpal response, and that this is also closely associated with its antibacterial properties.
Mineral trioxide aggregate
Mineral trioxide aggregate (MTA) is a cement composed of tricalcium silicate, dicalcium silicate, tricalcium aluminate, tetracalcium aluminoferrite, calcium sulphate and bismuth oxide. Its composition is not unlike that of Portland cement, except for the addition of bismuth oxide. The latter is added in order to improve its radiopacity.
MTA is very alkaline (pH ~12.5) and has biological and histological properties similar to those of calcium hydroxide cement. It has been shown that MTA can induce bone deposition with a minimal inflammatory response, as it is less cytotoxic than reinforced zinc oxide–eugenol cements.
The material is mixed with sterile water to provide a grainy, sandy mixture and then can be packed gently into the desired area. The material is not the easiest to handle, although some clinicians claim that calcium hydroxide cement is technically more difficult to place. The powder-to-liquid ratio (3 : 1) is critical if one is to achieve appropriate hydration of the powder. MTA requires moisture to set, such that absolute dryness not only is unnecessary but also is contraindicated. On occasion, it may be necessary to place a moist cotton pellet directly in contact with the material in order to allow proper setting; however, excessive moisture softens the material. It takes an average of 4 hours for the material to solidify completely and, once the cement has set, it has a compressive strength comparable to that of reinforced zinc oxide–eugenol cement. It should be noted that a low pH environment can prevent the material from setting.
MTA has also been recommended for use as a root-end-filling material, a retrograde root-filling material, to seal perforations or open apices, or to cap vital pulps.
Dentine-bonding agents
The use of dentine-bonding agents is even more controversial than the use of calcium hydroxide as a direct pulp-capping agent, but is an area being extensively researched. As stated by Stanley (1998), the research data on pulp capping are, at times, inadequate, confusing, misleading or even incorrect, and diminish the confidence of practitioners in performing pulp capping. At best, the situation is confusing and more research is needed to make any definitive statement. Haemostasis seems to be essential and, for this, cleaning with a dilute solution of sodium hypochlorite (1.0% or less) has been recommended. If bleeding cannot be controlled within a matter of a minute, endodontic treatment is indicated.
What continues to remain controversial is the practice of total-etch direct bonding with dentine-bonding agents, and more research needs to be focused on this area. Some successes have been claimed for direct pulp capping with dentine-bonding agents when the acid-etch step is omitted, or for bonding systems not requiring a separate acid-etch step, such as the self-etching primers, despite the observation that phosphoric acid can act as an effective haemostatic agent. Hence, the results obtained with one dentine-bonding agent may be different from those obtained with another dentine-bonding agent, such that clinical experience cannot be extrapolated from one to the other. It is, therefore, not surprising that most general dental practitioners continue to use a minimal quantity of calcium hydroxide, before placing a dentine-bonding agent.
Failure after direct pulp capping
Failure after direct pulp capping can be due to three reasons:
It is important to distinguish the last of these failures from the others, as it is not, strictly speaking, a failure of pulp capping. The outcome of direct pulp capping with calcium hydroxide cement can be unpredictable, which may be related to the importance of achieving direct contact between the sealant and the pulp tissue without any intervening blood clot. It has been found that iatrogenic pulp exposures treated with MTA are generally free from inflammation 1 week after placement and a hard tissue bridge will form over a period of about 3 weeks. Thus it would appear that MTA has excellent sealing abilities and prevents pulpal inflammation by providing a predictable secondary barrier under the surface seal provided by the restorative material.
Root canal filling materials
The objectives of modern non-surgical endodontic treatment are:
A wide variety of materials have been used in an attempt to produce an impervious seal of the tooth root apex. The most widely used root-canal-sealing materials are a combination of root-obturating points and canal-sealer cements.
Obturating points
Gutta percha
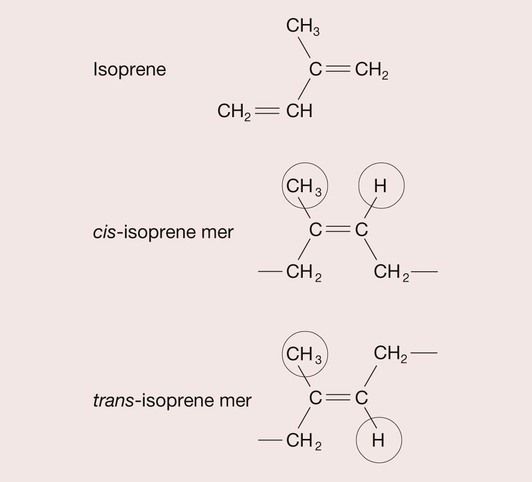
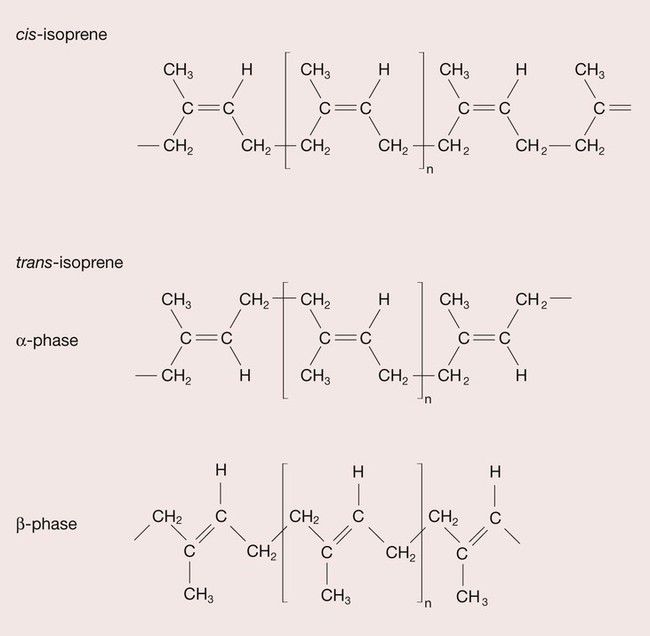
Gutta percha is a rubber that is tapped from the Taban tree. It was introduced into the UK in 1843 and has been used in endodontics for over 100 years. Rubbers are polymers of isoprene (2-methyl-1,3-butadiene), and isoprene is a geometric isomer, which means that it can have different structural arrangements despite having the same composition, as depicted in Figure 2.6.3. When the CH3 group and the H atom are positioned on the same side of the isoprene mer, this is termed a cis structure and the resulting polymer, cis-isoprene, is known as natural rubber. When the CH3 group and the H atom sit on opposite sides of the isoprene mer, this is termed the trans structure and trans-isoprene polymer is commonly referred to as gutta percha (Figure 2.6.4). The effect on the properties of these different configurations of the polymer is quite profound. In the cis form, the hydrogen atom and methyl group prevent close packing such that the natural rubber is amorphous and consequently soft and highly flexible, whereas the gutta percha crystallizes, and usually becomes about 60% crystalline, forming a hard, rigid polymer.
The natural rubbers are soft and tacky unless they are hardened by vulcanization, a process discovered by Charles Goodyear in 1839 (see page xii). Vulcanization involves heating the polymer with a few percent by weight of sulphur. The hardening occurs because sulphur bridges or cross-links form between the polymer chains, preventing the polymer molecules from slipping over one another. This cross-linked rubber is used to produce rubber dam and rubber gloves.
Gutta percha is a thermoplastic material and softens at 60–65°C; it will melt at about 100°C, so it cannot be heat-sterilized. If necessary, disinfection can be carried out in a solution of sodium hypochlorite (5%). The use of solvents such as acetone or alcohol should be avoided, as these are absorbed by the gutta percha, causing it to swell. Eventually, the gutta percha will return to its unswollen state, thus compromising the apical seal. On exposure to light, gutta percha oxidizes and becomes brittle. It is therefore important to check that the points have retained their flexibility before using them.
The gutta percha is able to take up two distinct conformations. At high temperature, the gutta percha chains take on an extended conformation, which can be preserved if cooled rapidly so that it forms the crystalline β-phase, whereas when the gutta percha is cooled more slowly, the denser α-phase is formed (see Figure 2.6.4). The α-phase gutta percha has better thermoplastic characteristics and is therefore preferred for use in hot gutta percha application systems, where heat-softened gutta percha is injected into the root-canal filling. This technique was first developed by Johnson in 1978 and further improvements of the original technique include the use of plastic carriers (Thermofil) and injection guns (Obtura) for the delivery of softened gutta percha. However, in the presence of a patent apical foramen, there may be a predisposition for extrusion of filling material beyond the apex.
An alternative approach is to dissolve the gutta percha in a chemical solvent such as chloroform or xylene. This softens the gutta percha and allows it to be adapted closely to the canal wall and duplicate the intricate canal morphology. However, as the solvent is lost, so the dimensional stability may be compromised, and concerns have been expressed regarding the possible cytotoxic effects of using these solvents.
One of the main uses is the gutta percha point, which is softened and com/>
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses