Immunity, inflammatory disorders, immunosuppressive and anti-inflammatory agents
Key Points
• Autoinflammatory disorders are increasingly recognized
• Anti-inflammatory therapies are more commonly used and directed at cytokines
• Immunosuppressive therapy has many beneficial effects but predisposes to infections and neoplasia
This chapter discusses innate and acquired immunity and inflammation, autoinflammatory disorders and immunosuppressive therapy.
Innate Immunity and Inflammation
Innate immunity includes basic mechanisms of resistance to infection, such as epithelial anatomical barriers of skin and mucous membranes, cilia, secretions such as saliva and tears, and the innate inflammatory response. The latter is characterized by increased localized blood flow and capillary permeability (inflammation), releasing soluble factors that recruit phagocytes (neutrophils and macrophages), which restrict and engulf invasive microorganisms.
Inflammation is initiated and maintained by mediators, especially complement and cytokines – small proteins that specifically also affect the behaviour of immune cells (immunocytes). Cytokines, including interleukins, lymphokines and cell signal molecules such as tumour necrosis factors and interferons, regulate the intensity or duration of immune responses by stimulating or inhibiting proliferation of various immunocytes, or by modulating their secretion of other cytokines or antibodies.
Complement
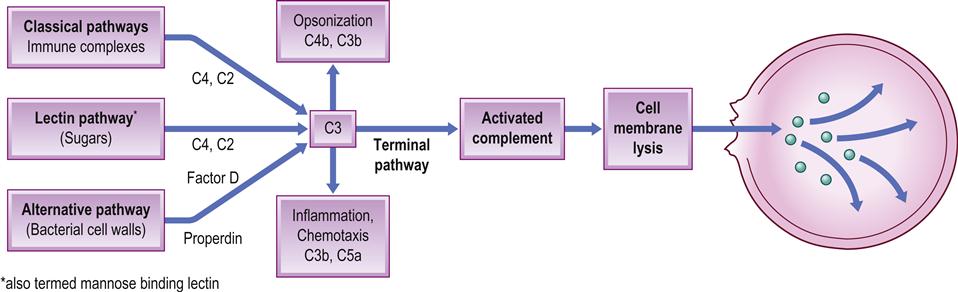
The complement system is a plasma protein sequence (cascade) involving at least nine plasma proteins. When activated, this sequence releases important mediators of inflammation and chemotaxis (C3a and C5a), compounds capable of opsonizing microorganisms for phagocytosis (C3b), and an attack complex (C5–9) capable of damaging cell membranes (Fig. 19.1). There are three complement pathways – classical, lectin and alternative – that differ in the manner in which they are activated (triggering agents include bacterial lipopolysaccharides [LPS] and immune [antigen/antibody] complexes), though all produce C3 convertase – the key enzyme. The system is controlled by inhibitors such as C1 esterase inhibitor, decay accelerating factor (DAF), membrane inhibitor of reactive lysis (MIRL) and homologous restriction factor (HRF).
Cytokines
Cytokines are a family of proteins – interferons, tumour necrosis factors, transforming growth factors, interleukins, chemokines and colony-stimulating factors – that act in concert with inhibitors and soluble receptors to regulate immune responses.
Interferons (IFNs) are one of the most important natural defences against viral infection. IFN-α and IFN-β are antiproliferative for virally infected and other cell types. IFN-γ is involved in cell-mediated immune responses, via Th1 lymphocytes; is antagonistic to interleukin 4 (IL-4); activates macrophages; and induces expression of class II major histocompatibility complex (MHC) antigens.
Tumour necrosis factors (TNFs) are important in inflammation and antitumour immunity. TNF-α is secreted by a variety of cells, including T and B lymphocytes, macrophages, natural killer (NK) cells, endothelial cells and keratinocytes. TNF-α primes both endothelial cells and neutrophils for adhesion, is chemotactic for inflammatory cells and can signal cells to begin apoptosis (programmed cell death). It is a death activator, triggering the proteolytic caspase cascade that culminates in apoptosis. It can also activate nuclear factor kappa B (NF-κB) and activator protein 1 (AP-1), leading to the induction of several genes and inflammation. It can also initiate and perpetuate an inflammatory response through upregulating adhesion molecules on microvascular endothelial cells, and enhance expression of MHC class I and II antigens, and co-stimulatory molecules on dendritic cells (DCs) and macrophages. Matrix metalloproteinase (MMP) production by stromal cells is induced, which may lead to tissue remodelling and the enhanced TNF-mediated secretion of keratinocyte growth factor (KGF). TNF can also induce fever, either directly via stimulation of prostaglandin E2 (PGE2) synthesis by the hypothalamic vascular endothelium, or indirectly by inducing release of the pyrogen interleukin 1 (IL-1), and can stimulate collagenase and PGE2 production. TNF also induces hepatic acute-phase reactant protein production.
TNF-β – from T and B cells, NK cells and astrocytes – mediates fibroblast and endothelial cell proliferation, important in wound healing.
Transforming growth factor beta (TGF-β) is part of a protein superfamily that includes inhibins, activins and bone morphogenetic protein (BMP), which can affect development, haemopoiesis, wound healing and tissue repair. TGF-β has a crucial role in cell-cycle regulation and apoptosis, via the SMAD (name derived from a fusion of gene names sma [in Caenorhabditis elegans] and Mad [in Drosophila]) or the DAXX (death domain-associated protein) pathways.
Interleukins and their functions are diverse. Over 33 interleukins have been identified, most originating from T cells, some macrophages and mast cells, or from B cells or other cells (Appendix 19.1).
Cytokines that promote inflammation are termed proinflammatory cytokines; others are anti-inflammatory (Table 19.1).
Table 19.1
| Proinflammatory cytokines | Anti-inflammatory cytokines |
| Tumour necrosis factor (TNF-alpha) | IL-4, IL-10 and IL-11 |
| Interferons (IFNs) | |
| Alpha | |
| Beta | |
| Interleukins (ILs) | |
| 1 | |
| 6, 15, 17 and 18 |
Chemokines (chemoattractant cytokines)
Historically known under names such as the SIS family of cytokines, SIG family of cytokines, SCY family of cytokines, platelet factor-4 superfamily or intercrines, chemokines are classified into four groups (Box 19.1).
Colony-stimulating factors (CSFs) are glycoproteins that bind to haemopoietic cell receptors to drive differentiation. Those most relevant to the immune response are shown in Box 19.2.
Acute-Phase Response
In response to injury, infection, physical trauma or malignancy, neutrophils and macrophages secrete several cytokines (most notably IL-1, IL-6, IL-8 and TNF-α), which cause the liver to produce acute-phase reactants – proteins whose plasma concentrations increase in response to, and suppress, inflammation (Table 19.2).
Table 19.2
| Protein | Main functions |
| Alpha1-antichymotrypsin | A serpin, which downregulates inflammation |
| Alpha1-antitrypsin | A serpin, which downregulates inflammation |
| Alpha2-macroglobulin | Inhibits coagulation and fibrinolysis |
| Caeruloplasmin | Inhibits microbial iron uptake |
| Complement | See text |
| C-reactive protein (CRP) | Binds to phosphorylcholine in bacterial membranes and phosphatidyl ethanolamine in fungal membranes activating the classical complement pathway |
| D-dimer protein | Fibrin degradation product |
| Factor VIII | Coagulation factor |
| Ferritin | Inhibits microbial iron uptake |
| Fibrinogen | Coagulation factor |
| Haptoglobin | Inhibits microbial iron uptake |
| Mannan-binding protein (MBP) or lectin (MBL) | Binds to microbial mannose-rich glycans to act as an opsonin, and also activates the lectin pathway |
| Plasminogen | Coagulation factor |
| Prothrombin | Coagulation factor |
| Serum amyloid A | Recruits immunocytes to inflammatory sites |
| von Willebrand factor | Coagulation factor |
C-reactive protein (CRP) and serum amyloid A protein (SAA) are critical in the innate immune response. CRP can bind to some bacteria and fungi, activating complement and inflammation, as can SAA, which can also stimulate macrophages to phagocytose debris. Most organisms that tend to be invasive are ingested by phagocytes, including blood neutrophils (polymorphonuclear neutrophilic leukocytes) and tissue macrophages (‘phage’=eating up), which kill most organisms. However, some (i.e. all viruses, and bacteria such as those causing tuberculosis or brucellosis) survive inside cells, including phagocytes, and then the immune system is the main defence. Clinically, this positive acute-phase response appears within 12 hours and can be measured by determining CRP levels or the erythrocyte sedimentation rate (ESR). The acute-phase response is also characterized by leukocytosis, fever, and alterations in the metabolism of many organs. In contrast, there is a negative acute-phase response with decreases in albumin, transferrin, transthyretin and insulin growth factor I.
Acquired Immune Response
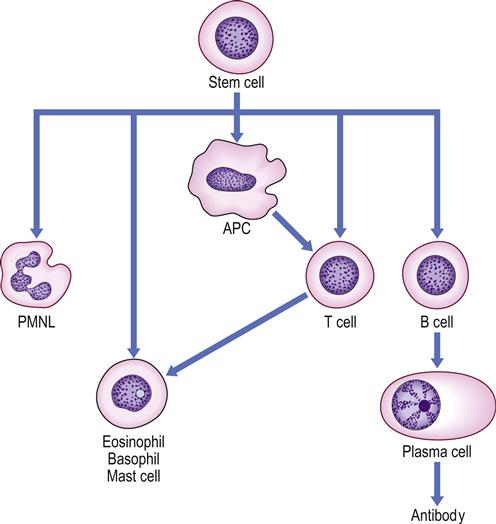
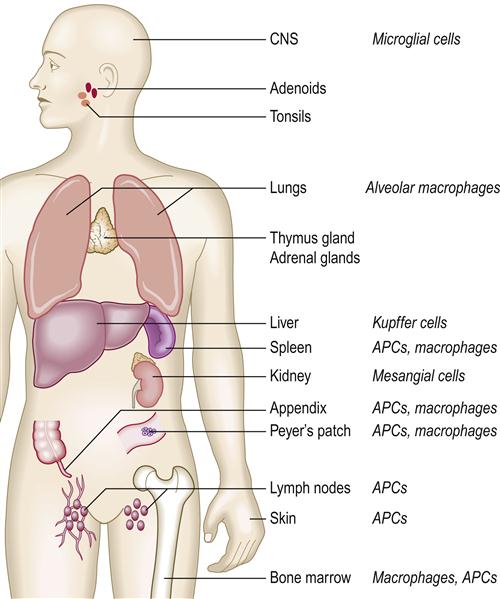
The main activity of the immune system is protection against infections. Immunocytes (leukocytes) are mainly lymphocytes and they produce humoral (antibody; mainly B-cell) or cell-mediated (mainly T-cell) responses that are central to the immune defences. B lymphocytes evolve into plasma cells, which produce antibodies to protect against extracellular organisms (Fig. 19.2). Antigens are processed by antigen-presenting cells (APC) and presented to T lymphocytes, which fulfil most other immune functions (i.e. cell-mediated immunity [CMI]). Macrophages and polymorphs are the main professional phagocytes; macrophages and macrophage-like cells are widely distributed in the lymphoreticular (reticuloendothelial) system (Fig. 19.3).
More than 70 types of leukocyte are recognized and defined by their cluster of differentiation (CD) antigens, which in turn are recognized by monoclonal antibodies.
Immunocytes are, in large part, regulated by cytokines produced by a variety of cells. Cytokines produced by lymphocytes are termed lymphokines, and those that act between leukocytes are called interleukins (see Appendix 19.1). Cytokines may also induce systemic effects, such as the acute-phase response (see above).
The movement of leukocytes between the blood and tissues depends on leukocyte–endothelial cell adhesion molecules known as selectins, integrins and the immunoglobulin gene superfamily (IgSF; intercellular adhesion molecules [ICAMs] and lymphocyte functional antigen 3), and over 20 other proteins.
Humoral Immunity
Antibodies are immunoglobulins. B cells carry surface immunoglobulins and also receptors for immunoglobulin G (IgG; CD32) and complement-activated components C3d (CD21) and C3b (CD35). Antibody production is modulated by T lymphocytes, which either assist (T-helper cells) or moderate (T-suppressor cells). The many cytokines involved include IL-1 to IL-7 and B-cell growth factor (BCGF). Immunoglobulins (antibodies) are of different classes (Table 19.3).
Table 19.3
| Classes | Comments |
| IgA | Secreted by exocrine glands and helps to protect mucosal surfaces |
| IgD | Of unclear function |
| IgE | Important in the mediation of atopic allergy but has a role in the defence against parasites |
| IgG IgM |
Essential for protection against bacterial infections, by such functions as neutralizing toxins, activating complement or promoting phagocytosis (opsonization) |
Complement and polymorphonuclear leukocytes (PMNLs) are essential to phagocytosis and inflammation. Cell-mediated responses are usually protective, and macrophages are also phagocytic.
Phagocytes and other Cells
Macrophages and dendritic cells intimately involved in antigen processing and the transference of information to lymphocytes are called antigen-processing cells (APCs). Phagocytes (PMNLs and macrophages) are attracted towards antigens, via activated complement after an antigen–antibody reaction, and can ingest and often kill microbes that are opsonized (i.e. coated by specific antibody and activated complement components). During phagocytosis or attempted phagocytosis of, for example, immune complexes, these phagocytes may discharge degradative enzymes (lysosomal enzymes), which can cause local tissue damage.
Large granular lymphocytes (LGLs; or null cells) are non-phagocytic cells that mediate NK cell activity and antibody-dependent cellular cytotoxicity (ADCC) – the binding and lysis of antibody-coated target cells. NK cells recognize malignant or foreign cells by a non-immune mechanism.
Basophils and mast cells have surface receptors for IgE, and contain histamine, prostaglandins, leukotrienes and proteases. They are involved in immune responses to parasites and in the immediate type of hypersensitivity responses (Ch. 17).
Prostaglandins (Table 19.4) and leukotrienes (Table 19.5) are produced from eicosanoids – lipids produced by the oxygenation of arachidonic acid from omega-3 fatty acids in fish oils, or released from cells by phospholipase A2. The enzyme cyclo-oxygenase (COX) produces prostaglandins and thromboxane, while the enzyme 5-lipoxygenase produces leukotrienes.
Table 19.4
Important prostaglandins (PGs)
| Prostaglandin | Sites of origin | Effects |
| PGD2 | Mast cells | Bronchoconstriction, vasodilatation and inhibition of platelet aggregation |
| PGI2 (prostacyclin) | Endothelium | Vasodilatation and inhibition of platelet aggregation |
| PGE2 | Epithelium, dendritic cells | Inhibition of lymphocyte proliferation, cytokine production and neutrophil function |
Table 19.5
| Leukotriene | Causes |
| LTB4 | Neutrophil chemotaxis, mucus secretion, cell growth |
| LTC4 (formerly termed slow-reacting substance A) | Vascular permeability, smooth muscle contraction, mucus production |
| LTD4 | |
| LTE4 |
Cell-Mediated Immunity
Antigens are processed by antigen-presenting cells, such as macrophages and Langerhans cells in epithelia, and presented to T cells in association with class I or II MHC molecules. T lymphocytes originate in bone marrow but differentiate within and are under the control of the thymus (hence T), acquiring immunological competence there, a process that requires the normal functioning of purine metabolism. T cells all have T-cell receptors, highlighted by the CD3 surface marker. When activated by antigens, T lymphocytes produce lymphokines, which, among other activities, can modulate nearby cells, particularly macrophages, resulting in CMI (type IV immune responses). These are particularly important in the defence against some intracellular bacteria, such as mycobacteria, and against viruses and fungi, graft rejection, graft-versus-host reaction, delayed hypersensitivity and defences against cancer cells.
Circulating T lymphocytes differentiate into CD4 cells – mainly helper T cells that are antigen-processing and can recognize MHC class II antigens, and can induce B-cell differentiation, induce CD8 cytotoxic T-cell proliferation, produce various soluble mediators (lymphokines) and regulate erythropoiesis. Healthy adults usually have CD4+T-cell counts of 1000 or more per mm3 of blood. CD4 helper T cells are either Th1 or Th2. Th1 cells secrete IL-2 and IFN-γ; they provide help for the generation of cytotoxic T cells involved in type IV immune responses (Ch. 17) and are suppressed by IL-10. Th2 secrete IL-4, 5, 6 and 10, which both help B cells to produce IgG, IgA and IgE, and regulate the production of other cytokines – and thereby the immune response. Th2 cells are involved in type II and type III immune reactions and are suppressed by interferon.
Circulating T lymphocytes can also differentiate into CD8 cells, which recognize MHC class I antigens and are one type of cytotoxic or suppressor T cell; they are important in eliminating virus-infected cells.
To summarize, T-cell proliferation and differentiation are regulated by many cytokines, including interleukins, interferons, tumour necrosis factors and transforming growth factors. Cytokines generally function as local signals for cell growth, differentiation, activation, inhibition, apoptosis or chemotaxis, but may also induce systemic effects such as acute-phase responses (fever, CRP, osteoclast activation, platelet release) induced by IL-1, IL-6 and TNF.
Evaluation of B-Lymphocyte Function
The initial screening for B-lymphocyte function is an assay of serum IgG, IgA and IgM. There are four subclasses of IgG, and selective deficiencies of these can develop. Thus, when there is a strong suspicion of a humoral immunodeficiency based on clinical grounds but the total IgG is normal, quantitative measurement of individual subclasses is indicated.
Assessment of antibody function is necessary. Antibody titres after immunization with protein antigens (e.g. tetanus or diphtheria toxoids) and polysaccharide (e.g. pneumococcal capsular polysaccharides) are most convenient (immunization with live viral vaccines must be avoided, as the person may be immunocompromised). If immunoglobulin levels and/or antibody titres are low, more advanced tests of B-lymphocyte numbers and function are needed.
Evaluation of T-Lymphocyte Function
Indirect information about T-lymphocyte function may be obtained by counting peripheral blood T lymphocytes. The total number of T lymphocytes (CD2 or CD3), T-helper lymphocytes (CD4) and T-suppressor/cytotoxic lymphocytes (CD8) in peripheral blood can be quantified with appropriate monoclonal antibodies.
Delayed-type hypersensitivity (DTH) skin tests use a panel of ubiquitous antigens to screen for T-cell function. A positive test generally indicates intact T-cell function and CMI, but requires prior exposure and sensitization to the antigen, which may not have happened. Also, a positive DTH skin test to some antigens does not guarantee that the patient will have normal CMI to all. Furthermore, some normal individuals may have transiently depressed DTH reactions during viral infections. Skin testing for DTH measures not only T-lymphocyte responses but also the afferent (receptor) arc of the cell-mediated immune response. Thus, most adults show DTH to tuberculin in the tuberculin (Mantoux) test, since they have had previous contact with Mycobacterium tuberculosis or bacilli Calmette–Guérin (BCG). A positive response in apparently well people is generally an index of ‘waiting immunity’ to the disease and usually does not mean that mycobacteria are actively causing cell-mediated tissue damage. A recent change from negative to positive in the absence of vaccination implies recent exposure, and prophylactic treatment may be indicated. By contrast, a negative tuberculin reaction indicates susceptibility to infection or occasionally that overwhelming infection is established – all CMI has been used up in an attempt to combat the infection. In such cases, successful treatment may allow CMI to return and a negative Mantoux test can then become positive.
It is possible also, in specialized laboratories, to measure several different cytokines involved in T- and B-lymphocyte regulation (e.g. IL-2, IFN-γ).
Evaluation of Phagocytic Function
A white blood cell count and differential are the first essentials. Assessment of phagocyte function requires in vitro assays of directed cell movement (chemotaxis), ingestion (phagocytosis) and intracellular killing (bactericidal activity).
Evaluation of the Complement System
Most complement components can be detected using antibody- sensitized sheep erythrocytes in a total haemolytic complement assay (CH50 assay), since this assay requires the functional integrity of C1 to C9. Deficiencies of alternative pathway components factors D, H and I and properdin can be detected by a haemolytic assay using activators of the alternative pathway, such as unsensitized rabbit erythrocytes. Individual components are detected by specialized functional and immunochemical tests.
Autoinflammatory Diseases (Periodic Syndromes)
General aspects
The autoinflammatory diseases (periodic syndromes) are rare disorders of innate immunity, often hereditary, which involve an ongoing imbalance between factors promoting and those inhibiting inflammation. The abnormalities seen include aberrant responses to pathogen-associated molecular patterns (PAMPs), including lipopolysaccharide (LPS) and peptidoglycan; neutrophilia; and dysregulation of the proinflammatory cytokines IL-1β and/or TNF-α, or the receptors for these cytokines.
Clinical features
Periodic syndromes usually begin from childhood and are characterized by fluctuating or recurrent episodes of fever and generalized inflammation, affecting the surfaces, joints, eyes and/or skin.
Patients with the autoinflammatory syndromes generally are well between abrupt episodes of fever and systemic inflammation, but some develop amyloidosis.
General management
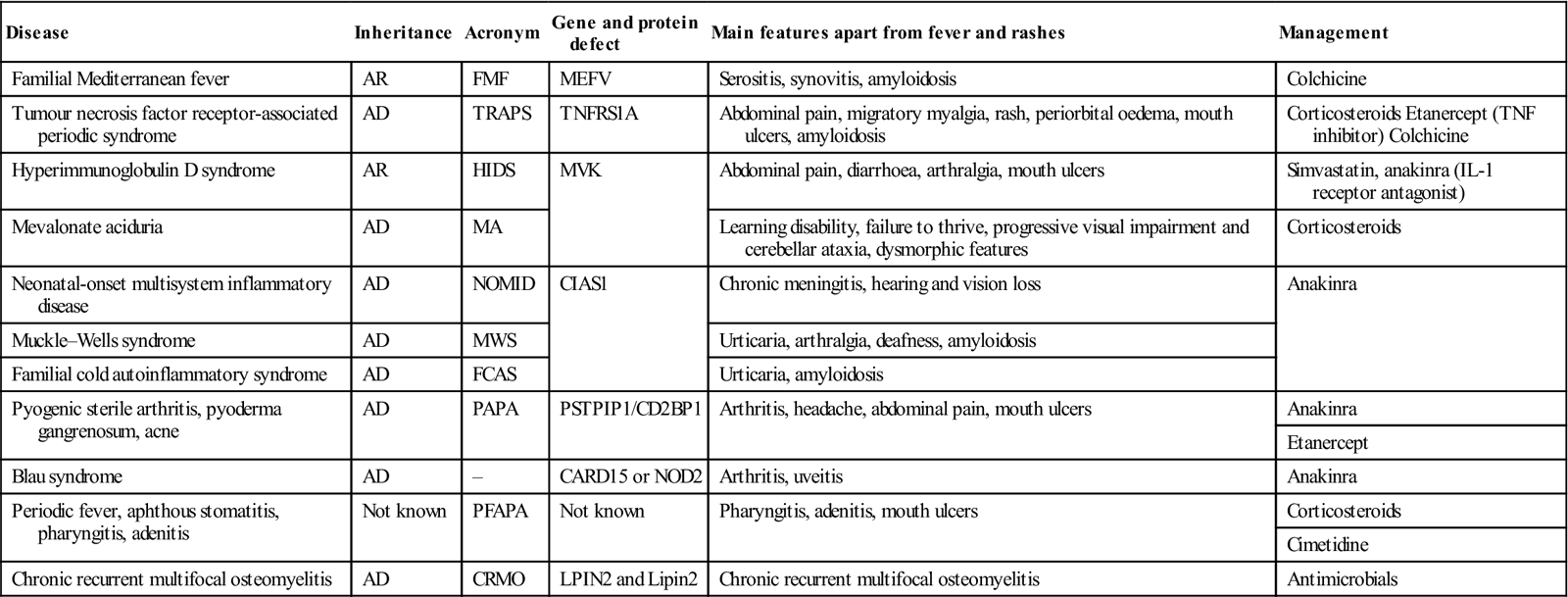
Diagnosis is supported by evidence of elevated acute-phase response during attacks, and often a positive family history and ethnic background. Genetic testing is indicated. Many of the syndromes have a specific treatment (Table 19.6).
Table 19.6
Main autoinflammatory syndromes
< ?comst?>
| Disease | Inheritance | Acronym | Gene and protein defect | Main features apart from fever and rashes | Management |
| Familial Mediterranean fever | AR | FMF | MEFV | Serositis, synovitis, amyloidosis | Colchicine |
| Tumour necrosis factor receptor-associated periodic syndrome | AD | TRAPS | TNFRS1A | Abdominal pain, migratory myalgia, rash, periorbital oedema, mouth ulcers, amyloidosis | Corticosteroids Etanercept (TNF inhibitor) Colchicine |
| Hyperimmunoglobulin D syndrome | AR | HIDS | MVK | Abdominal pain, diarrhoea, arthralgia, mouth ulcers | Simvastatin, anakinra (IL-1 receptor antagonist) |
| Mevalonate aciduria | AD | MA | Learning disability, failure to thrive, progressive visual impairment and cerebellar ataxia, dysmorphic features | Corticosteroids | |
| Neonatal-onset multisystem inflammatory disease | AD | NOMID | CIAS1 | Chronic meningitis, hearing and vision loss | Anakinra |
| Muckle–Wells syndrome | AD | MWS | Urticaria, arthralgia, deafness, amyloidosis | ||
| Familial cold autoinflammatory syndrome | AD | FCAS | Urticaria, amyloidosis | ||
| Pyogenic sterile arthritis, pyoderma gangrenosum, acne | AD | PAPA | PSTPIP1/CD2BP1 | Arthritis, headache, abdominal pain, mouth ulcers | Anakinra |
| Etanercept | |||||
| Blau syndrome | AD | – | CARD15 or NOD2 | Arthritis, uveitis | Anakinra |
| Periodic fever, aphthous stomatitis, pharyngitis, adenitis | Not known | PFAPA | Not known | Pharyngitis, adenitis, mouth ulcers | Corticosteroids |
| Cimetidine | |||||
| Chronic recurrent multifocal osteomyelitis | AD | CRMO | LPIN2 and Lipin2 | Chronic recurrent multifocal osteomyelitis | Antimicrobials |
< ?comen?>< ?comst1?>
< ?comst1?>
< ?comen1?>
Dental aspects
Aphthous-like oral ulceration has been reported as one manifestation in periodic fever, aphthous stomatitis, pharyngitis, adenitis (PFAPA); familial Mediterranean fever (FMF); hyperimmunoglobulinaemia D and periodic fever syndrome; tumour necrosis factor receptor-associated periodic syndrome (TRAPS); and pyogenic sterile arthritis, pyoderma gangrenosum, acne (PAPA).
Chronic recurrent osteomyelitis has been recorded in the jaws in chronic recurrent multifocal osteomyelitis. There is a suggested association of FMF with Behçet disease.
Periodic Fever, Aphthous Stomatitis, Pharyngitis, Adenitis (Marshall Syndrome)
PFAPA is not a familial disease. Attacks usually appear in pre-school children as recurrent fever with aphthous-like stomatitis, pharyngitis and cervical lymphadenitis. Long-term sequelae have not been reported and most children eventually grow out of it. Diagnostically, raised ESR, leukocyte count, fibrinogen and serum IgD have been found. PFAPA often responds well to corticosteroids, cimetidine or tonsillectomy.
Deficiency of The Interleukin-1 Receptor Antagonist (DIRA)
Deficiency of interleukin-1 receptor antagonist IL-1Ra (DIRA) is caused by inherited mutations in the gene IL1RN that encodes IL-1Ra. This antagonist binds to the same cell receptors as does IL-1 and blocks its inflammatory actions, so without IL-1Ra, the IL-1–induced systemic inflammation is uncontrolled. Mutations causing DIRA are rare, but more common in people of Puerto Rican or Dutch descent. Features include serious and potentially fatal bone swelling, pain and deformity; periosteitis; and skin pustulosis. Most children develop features from birth to 2 weeks of age and most respond to anakinra.
Deficiency of the Interleukin-36–Receptor Antagonist
Aberrant interleukin-36Ra structure and function cause secretion of proinflammatory cytokines, hyperleukocytosis and raised C-reactive protein and generalized pustular psoriasis.
Immunosuppressive Therapy
See also Chapter 35.
Where inflammation can become harmful, attempts can be made to suppress the immune response or block cytokine action. Most immunosuppressive drugs predominantly depress cell-mediated responses and autoantibody production more strongly than normal antibody production.
Immunosuppressive therapy is widely used, especially to suppress rejection in transplant recipients (bone marrow, kidney, liver, pancreas, heart and heart–lung; Ch. 35), to treat autoimmune and connective tissue diseases, and to control tumours – especially lymphoproliferative neoplasms (Ch. 8).
Immunosuppressive therapy inevitably predisposes patients significantly to infections, particularly viral, fungal, mycobacterial and protozoal ones, since T-cell immunity is largely depressed; patients are also predisposed to malignant disease (Kaposi sarcoma, lymphomas and squamous cell carcinomas of the skin, cervix and lip), most of which are virally related. Infections may be opportunistic (i.e. involve microorganisms that are normally commensal), may spread rapidly and often may be clinically silent or atypical.
Immunosuppressive strategies now attempt to be more selective in their effects on the immune response by using biological agents (also termed targeted immune modulators [TIMs]) and biological response modifiers [BRMs]) designed to inhibit the harmful effects of upregulated proinflammatory cytokines (e.g. TNF-α, IL-1 and receptor, IFN-β, IFN-α, IL-6, IL-15, IL-17 and IL-18). Potential therapeutic strategies may augment the activity of anti-inflammatory cytokines (e.g. IL-4, IL-10 and IL-11) (see below).
Systemic non-biological immunomodulators are used in oral health care in mucosal disease:
■ Severe, resistant or systemic lesions in:
aphthae or aphthous-like ulceration
They are also used in other conditions:
Most affect cytochrome P450-3A4 and P-glycoprotein drug pathways, and interact with foods/medications. All may have serious adverse effects, including:
Systemic Non-Biological Immunomodulators
Drugs such as azathioprine, cyclophosphamide and chlorambucil are cytotoxic to cells, including some immunocytes; they thus act as fairly crude immunosuppressive agents and adverse effects are common (Appendix 19.2). Calcineurin inhibitors bind to immunophilins (cyclophilin and macrophilin) and block receptor expression, T-cell activation and cytokine release. Calcineurin inhibitors (ciclosporin and tacrolimus) may enhance tumour development through mechanisms independent of host immunity. In contrast, inhibitors of the mammalian target of rapamycin (mTOR), including sirolimus and everolimus, are newer immunosuppressants that have antineoplastic properties.
Biologics (Biological Response Modifiers)
(Appendix 19.3; see also Chapter 35.)
Biologics are produced mainly by recombinant DNA technology and are usually:
Biological response modifiers (BRMs) block the inflammatory and immune responses, acting on immunocytes directly or via cytokines, inhibiting cellular activation and inflammatory gene transcription by various means.
Some are antibodies, soluble receptors or natural antagonists; others are small molecules that specifically inhibit intracellular, cell–cell and cell–matrix interactions intrinsic to inflammatory and immune processes. Examples are shown in Appendices 19.3 and 19.4.
Biologic therapies aim to modulate lymphocytes or cytokines. They include:
TNFα inhibitors (etanercept, adalimumab, infliximab, golimumab, certolizumab, natalizumab) bind and⁄or neutralize soluble (both circulating and within tissue) and membrane-bound TNFα, so blocking its effects upon target inflammatory cells.
T-cell modulators act on specific CD antigens. Alefacept targets CD2+on memory T and NK cells. B-cell modulators, such as rituxim/>
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses