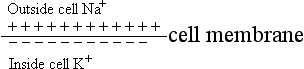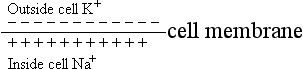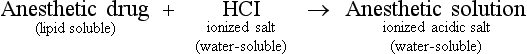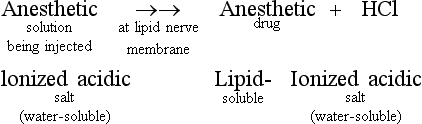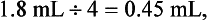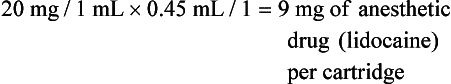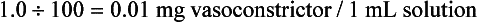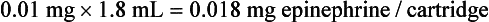Management of Pain and Anxiety
Characteristics and Physiology of Pain
1. Pain—sensation of discomfort resulting from the stimulation of specialized nerve endings called nociceptors (free nerve endings)
2. Pain perception—process whereby the sensation of pain is transmitted from the periphery to the central nervous system (CNS)
3. Pain reaction—result of pain perception; what a person will do about the perceived pain
4. Pain-reaction threshold—amount of pain one must experience before exhibiting a reaction
B Pain reactions and pain-reaction thresholds vary from individual to individual and within the same individual from day to day
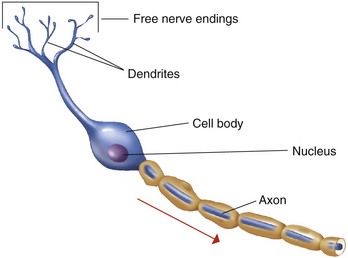
1. Functional unit—neuron, or nerve cell
3. Sensory neuron characteristics (Figure 18-1)
4. Fiber diameter—varies; determines speed of impulse conduction and type of pain perceived
5. Nerve trunk versus ganglia—a nerve trunk is an extremely large bundle of nerve fibers or axons; a ganglion is the site where cell bodies are bundled
1. Membrane potential—the difference in the electrical charges across the nerve membrane
2. Membrane potential maintained by:
3. Ions essential to nerve conduction
4. Sodium–potassium pump; resting state polarized (unstimulated)
5. Permeability of the cell membrane—impermeable to sodium (at rest)
1. Stimulus—an environmental change that can be chemical, thermal, mechanical, or electrical in nature
2. Minimal threshold stimulus—the magnitude of the stimulus required to initiate a nerve impulse
3. All-or-none law—either the nerve fires as strongly as possible or it does not fire at all
1. When a minimal threshold stimulus excites the nerve:
2. Depolarization—the time interval that exists while ionic concentrations are reversing
3. Reversed polarity—the result of a reversal in ionic charges
4. Repolarization—occurs after reversed polarity; the membrane becomes hyperpermeable to K+ and impermeable to Na+; polarity is re-established
5. Action potential—rapid sequence of changes in the membrane potential (negative to positive, and positive back to negative); the stages are:
6. Absolute refractory period—the period during depolarization and reversed polarity when the cell membrane cannot be re-excited
7. Relative refractory period—during repolarization, the nerve cell membrane can be re-excited, but it requires a greater stimulus than the stimulus required for excitation from the resting state
G Pain intensity determined by:
H Pain reaction—determined by a person’s pain reaction threshold
1. High pain reaction threshold produces hypo-reactive behavior
2. Low pain reaction threshold produces hyper-reactive behavior
3. Factors affecting the pain reaction threshold
a. Emotional state—most consistently reported variable; greater anxiety and negativity results in lower pain-reaction threshold
b. Fatigue—greater fatigue results in lower pain reaction threshold
c. Age—younger people experience a lower pain reaction threshold
Armamentarium
A Definition—all items essential for the administration of a local anesthetic agent
b. Composition—stainless steel
c. Gauge (ga)—the diameter of the lumen is indicated by a number
(1) The larger the gauge number, the smaller is the lumen diameter
(2) The 25-, 27-, and 30-ga needles are the most common in dentistry
d. Length—measured from hub to the point of bevel
e. Method of sterilization—disposable needles come presterilized, with a security seal
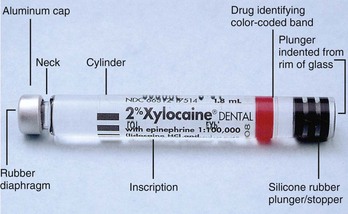
(1) Metal cap end is directed toward the needle when the cartridge is loaded into the syringe
(2) Round rubber diaphragm—the syringe end of the needle penetrates this diaphragm and enters the cartridge
(3) Cylinder—glass tube containing the anesthetic solution
(4) Inscription—legally required information on the cartridge, which includes:
(5) Rubber stopper—seals the opposite end of cartridge; it is the movable component of the cartridge
b. Ingredients in the anesthetic solution
c. Method of storage—away from direct sunlight and ultraviolet light, and maintained at room temperature (68°F to 77°F; 20°C to 25°C) or slightly cooler
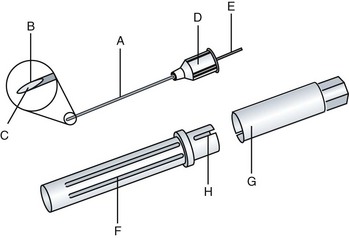
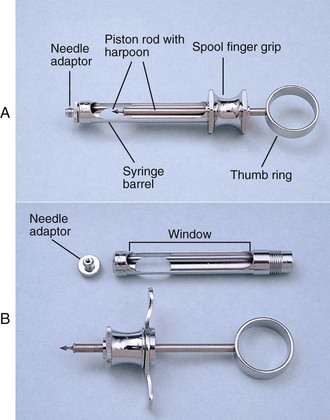
3. Anesthetic syringe (Figure 18-4)
(1) Adapter—the hub of the needle is threaded onto this part of the syringe
(2) Barrel—holds the anesthetic cartridge
(3) Harpoon—the fish-hook–shaped tip is forced into the rubber stopper
(4) Piston rod—connects the harpoon to the thumb ring; can be advanced to expel the solution or retracted to aspirate
(5) Large window—faces the operator during injection; rubber stopper speed, positive aspirations, and volume are observable
(6) Small window—aids in removing the used cartridge
(7) Spool finger grip—place of rest for the index and middle fingers
(8) Thumb ring—enables the operator to aspirate or express fluid from the cartridge
b. Method of syringe sterilization—the bioburden is removed; the syringe is dried, packaged, and sterilized
c. Auxiliary materials—hemostat or cotton pliers; used if the needle breaks
C Record keeping and documentation in client dental record
Local Anesthetic Agents
Chemical Structure
A Chemical components (Table 18-1)
1. Aromatic lipophilic group—for diffusion into the lipid nerve membrane
2. Intermediate chain—determines if the agent is an ester or an amide; also determines the potential for any allergic reaction
3. Hydrophilic group—required for diffusion into water-soluble tissues
B Allergies related to chemical structure
1. A considerable amount of documentation on allergic reactions to esters is available; because of their potential for causing allergic reactions, esters are no longer used
2. Cross-reactivity occurs with esters; thus, if a client is allergic to one ester, an allergic reaction will occur with all other ester derivatives
3. No substantiated cases, to date, of allergic reactions to amides
4. Cross-reactive allergic reactions do not occur among amides
5. In cases where the agent cannot be identified, the client should be referred to an allergist for testing
Mechanism of Action
A Physiologic effect—local anesthetics prevent cell membrane permeability to sodium
C Dissociation—the ability of a drug to separate or part
D Effects of inflammation—decreased anesthetic effect in areas of inflammation; caused by three factors:
Potency
Toxicity
A Definition—the amount of drug capable of causing adverse systemic reactions in normal persons; adverse reactions occur when the rate of drug absorbed is greater than the rate of biotransformation, the body’s ability to metabolize the drug
B True toxic reactions occur immediately—the most profound effects of toxic reaction are on the central nervous system (CNS) and the cardiovascular system
a. CNS stimulation phase—the person becomes extremely talkative, restless, and anxious; convulsions may occur
b. CNS depression phase—in extreme cases, unconsciousness may result
a. During the stimulation phase—the person’s blood pressure and pulse rise rapidly
b. During the depression phase—the person’s blood pressure and pulse drop significantly
3. Respiratory failure is the primary cause of death
4. Vital functions must be supported until the drug is eliminated by biotransformation (metabolism)
Biotransformation (Metabolism)
Topical Anesthetic
A Purpose—reduces the discomfort associated with the initial penetration of the needle through the mucosa
B Controversy exists with regard to widespread use
1. Dosage control—impossible to standardize because of variability in factors such as operator, client, and area of operation
2. The concentration of the topical anesthetic used exceeds that which is administered by injection (e.g., 5%, 10%, 22% topical anesthetic versus 2%, 3%, or 4% local anesthetic)
3. Except for 5% lidocaine (Xylocaine), all other topical anesthetics are esters
4. Lidocaine or prilocaine periodontal gel (Oraqix) is an amide; used in adults who require limited pain control during root debridement in periodontal pockets
5. Cetacaine—a combination of benzocaine, butamben, and tetracaine hydrochloride—is an ester topical indicated in adults who require pre-injection, deep scaling, and suture removal anesthesia (available in spray, liquid, or gel form)
C Esters have a greater incidence of allergic reactions and cross-reactivity than amides
D Topical agents have indications and contraindications; can interact with other medications; clinicians must always assess for potential allergies, side effects, and adverse effects and take appropriate precautions
Calculating the Amount of Local Anesthetic Drug in an Anesthetic Solution
A Maximum recommended dose (MRD)—maximum amount of drug administered that does not produce a toxic reaction
1. Percentage of the anesthetic drug is the number of grams (g) of drug per 100 milliliters (mL) of solution (e.g., 2 g anesthetic drug/100 mL solution = 2% anesthetic solution). To find the number of milligrams of drug in 1 milliliter of solution, change the grams of drug to milligrams (mg) by multiplying by 1000 (e.g., 2 g drug/100 mL solution × 1000 mg/1 = 2000 mg/100 mL = 20 mg drug/1 mL solution). This calculation determines that 20 mg of anesthetic drug (e.g., lidocaine) are in each milliliter of this anesthetic solution
2. To calculate the number of milligrams of anesthetic drug (e.g., lidocaine) that is administered, multiply the number of milligrams per milliliter of the drug by the number of milliliters of solution administered
a. Anesthetic cartridges used in dentistry contain 1.8 mL of solution; if an entire cartridge of solution was injected, the calculation would be as follows:
b. If only 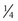 of 1 cartridge was administered, the calculation would be:
of 1 cartridge was administered, the calculation would be:
3. To compute the MRD of an anesthetic drug in cartridges, divide the MRD in milliliters by the number of milliliters in 1 cartridge (1.8). The MRD of lidocaine 2% is 15 milliliters (15 mL/1.8 mL = 8.3 cartridges)
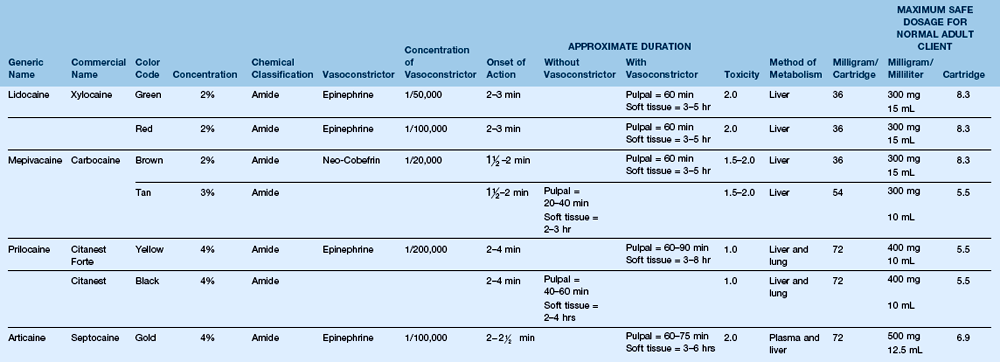
Vasoconstrictors (Table 18-2)
TABLE 18-2
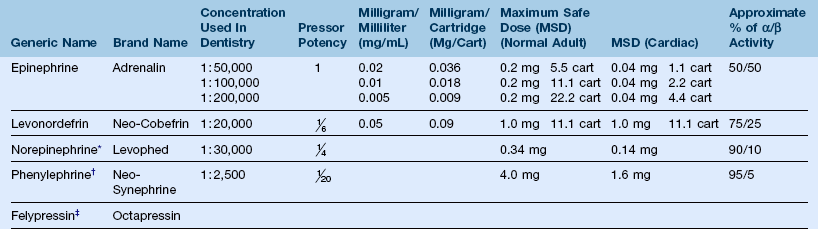
*Was included with procaine/propoxycaine (withdrawn in January 1996).
A Definition—one of five ingredients in an anesthetic solution that functions to hold the anesthetic agent in the area where anesthesia is delivered
B Description—all vasoconstrictors can be referred to as adrenergic drugs or sympathomimetic amines
1. Adrenergic drug—capable of producing the same effects as those of adrenalin
2. Sympathomimetic amine—mimics the sympathetic nervous system and contains an amine group within its chemical structure, which is characteristic of vasoconstrictors
3. All vasoconstrictors can be produced synthetically; two naturally occurring vasoconstrictors, epinephrine and norepinephrine, are produced in the adrenal medulla and the sympathetic postganglionic nerve fibers
C Function—hold the local anesthetic agent in the target site of analgesia by producing vasoconstriction; if absent from a local anesthetic agent, vasodilation will occur; the results of vasoconstriction are:
1. Reduction of systemic absorption of the local anesthetic agent
2. Reduction of chances of systemic toxicity
3. Reduction of blood flow through the area (hemostasis)
4. Prolongation of the action of the anesthetic
5. Increase the effectiveness of the local anesthetic by decrease in diffusion
6. Lower concentrations of the local anesthetic agents required
D Mode of action—stimulate α-receptors located in the walls of arterioles
1. α-receptors—responsible for arterial constriction
2. β-receptors—responsible for dilation
3. Each type of vasoconstrictor possesses varying degrees of response of both α- and β-activity (see Table 18-2); although epinephrine exhibits the most β-activity, to achieve the same amount of vasoconstriction as epinephrine, the concentration of all other vasoconstrictors must be increased
E Biotransformation—metabolism of vasoconstrictors occurs in the bloodstream; the enzyme responsible for biotransformation is monoamine oxidase (MAO); persons taking MAO inhibitors have a decreased ability to metabolize vasoconstrictors
F Pressor potency—the ability of a drug to produce vasoconstriction
1. Epinephrine—the most potent vasoconstrictor; pressor potency value = 1
2. Levonordefrin—another type of adrenergic vasoconstrictor; pressor potency value = 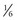
3. Concentrations of all vasoconstrictors must be increased to obtain the same vasoconstrictive potency as that of epinephrine
G Vasoconstrictors in solution—unstable; preservative (e.g., sodium bisulfite) is added to prevent oxidation
H Toxicity—toxicity resulting from vasoconstrictor overdose is caused by constriction of blood vessels, which raises blood pressure and cardiac rate from β-receptor stimulation
2. Prevention of cardiac emergencies—strict adherence to MRD in the case of persons with cardiac disease (see Chapter 21)
I Dosage calculations (maximum recommended dose); for example, epinephrine 1 : 100,000
1. 1 : 100,000 means 1 g epinephrine/100,000 mL solution
2. Multiply 1 g × 1000 to determine the number of mg/100,000 mL of solution
3. Divide the number of milliliters of solution by the number of milligrams of vasoconstrictor
4. If you administered more than 1 mL of solution, multiply the concentration in mg/mL by the number of mL administered
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses