Chapter 18 Dental bleaching systems
Learning Objectives
From this chapter, the reader will:
• Be aware of the various dental bleaching products
• Understand the risks and benefits of these products
• Have an appreciation of the various techniques that are currently used to lighten teeth
• Understand the legal position for the practice of tooth whitening in the UK
• Know the names of currently available commercial products.
Introduction
Over the past 20 years, the general public and therefore dental patients have become much more conscious of the appearance of their teeth. Their awareness of the treatment options dentists can offer has also increased owing to the increased media attention to dental health and cosmetic dentistry: there are many television programmes showing patient transformations using cosmetic surgery including cosmetic dentistry; and the public is bombarded with photographs of models with very white teeth in advertisements and glossy magazines. Although these photographs are very often ‘adjusted’ with the use of digital software tools, the perception that white, straight, perfect teeth are the norm and therefore desirable, has in many countries, contributed to a cultural shift, particularly in the USA. The market for cosmetic products and treatments has greatly increased, and it is now very common for patients to enquire about whitening their teeth during their dental appointment. Tooth bleaching is now the most commonly requested cosmetic service. Frequently, this appears in the patients’ minds to be more important than the treatment of dental disease. Beauty salons and hairdressers are also offering tooth whitening treatments although there are legal issues (in the UK) concerning non-dental care professionals carrying out any form of dental treatment.
Chemical Reaction: An Oxidizing Process
Mode of action
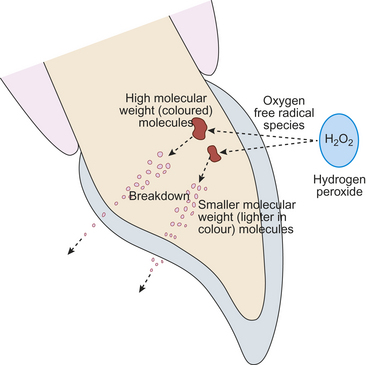
The free radical oxygen species break down the high molecular weight coloured complex organic molecules which cause staining. The smaller molecules so produced reflect less light from or they are lost from the tooth tissue with the result that the tooth tissue appears lighter in colour. Generally speaking, after an hour’s clinical use these breakdown products are rendered inactive (Figure 18.1).
Common Ingredients in Tooth Whitening Products
Besides the active ingredients discussed previously, several other chemicals are included in bleaching products by the manufacturers. See Table 18.1.
Table 18.1 Common chemical ingredients in tooth whitening products
| Chemical | Reason for inclusion |
|---|---|
| Hydrogen peroxide | Active ingredient |
| Carbamide peroxide | Source of hydrogen peroxide |
| Sodium perborate | Source of hydrogen peroxide |
| Urea | Stabilizer, and increases the pH, which is less irritant to soft tissue |
| Increased antibacterial effect | |
| Glycerine | Increases viscosity, so that the product is retained in the bleaching tray |
| Carbopol (polyacrylic acid polymer) | Increases viscosity, decreases breakdown in saliva and slows release of oxygen |
| Alcohol ethoxylates or sodium xylene sulphonate | Surfactant – promotes wetting by lowering surface tension or to solubilize one of the ingredients, such as an insoluble fragrance |
| Amorphous calcium phosphate (ACP) | Decreases sensitivity by occluding the dentinal tubules with calcium phosphate |
| Improves enamel smoothness and restores lustre | |
| Potassium nitrate | Decreases sensitivity by altering nerve conduction |
| Fluoride (e.g. sodium fluoride) | Decreases sensitivity by occluding the dentinal tubules |
| Promotes remineralization | |
| Provides caries resistance | |
| Neutralizers | Alkaline substances to create neutral pH |
| Flavourings | Increases patient acceptability |
| Carotene | Converts light energy to heat so increasing the activation of hydrogen peroxide by speeding up its dissolution into free radicals in products intended to be exposed to light energy |
Indications and Contraindications
• Children under 14 years of age
• Patients with kidney disorders or on kidney dialysis
• Patients allergic to hydrogen peroxide or other ingredients contained in the bleaching product
• Heavy smokers, unless they abstain from smoking for the duration of the treatment and subsequently
• Amalgam stains in tooth tissue. Such stains are resistant to bleaching
• Teeth displaying pathology such as dental caries, deficient restorations or periradicular disease.
Side Effects, Risks and Hazards
• (Transient) thermal sensitivity
• Gingival and soft tissue irritation
• Adverse structural changes in enamel
Thermal sensitivity
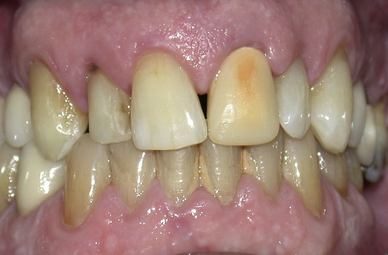
The most common side effect of bleaching treatments is sensitivity to thermal stimuli and occurs in between 25% and 50% of patients. As mentioned earlier, the oxygen species diffuse slowly through the hard dental tissues, eventually reaching the pulp. The volume reaching this site is dependent on the initial concentration and amount used. Many patients experience peak sensitivity on day 3 of treatment. The sensitivity tends to be transient and usually abates 2 days after discontinuation of treatment. Sensitivity has been reported more commonly in the lower than the upper arch, probably because of with relatively smaller size and volume of the tooth crowns. For this reason, many clinicians treat the upper arch before embarking on the lower. This also allows the clinician and patient to see what final result may be achieved (Figure 18.2). Long-term studies have shown no detrimental effects of this sensitivity after a period of 7 years.
Altered taste sensation
Some patients have reported a metallic taste during treatment. The strong oxidizing effect of the active species will cause mucosal changes and may have an effect on the tongue mucosa, altering the taste for short period of time. It may also be associated with a reaction between restoratives and the oxidizing agent. This is observed most commonly when the tray is removed in the morning after overnight bleaching. The taste usually disappears after a couple of hours. Many of the products used for bleaching are supplied in various flavours and the exposure to different flavours may also compromise the ability to taste other foods.
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses