Polymers for Prosthetic Dentistry
Chapter Objectives
On completion of this chapter, the student will be able to:
1. Describe the formation of long-chain polymers from monomers.
2. Explain the effect that cross-linking has on the physical properties of polymers.
3. Describe the stages of addition polymerization.
4. Explain the function of a free radical.
5. List the important properties of acrylic resins.
6. Describe the procedure for heat processing a denture.
7. Explain the importance of control of heat and pressure when processing a denture.
8. Explain the differences between hard and soft lining materials.
9. List the indications for long- and short-term soft liners.
10. Describe the advantages and disadvantages of chairside and laboratory hard liners.
11. List the indications for the use of acrylic teeth versus porcelain teeth.
12. Describe the process for repairing acrylic dentures.
13. Describe the use of the ultrasonic cleaner for cleaning complete and partial dentures in the office.
14. Describe the home care regimen for complete and partial dentures that patients should follow.
15. List the precautions patients should take when cleaning their dentures.
16. Understand the steps for fabrication of custom acrylic impression trays.
Key Terms defined within the chapter
Polymerization the act of forming polymers; the process can be activated by chemicals, heat, or light
Cross-Linked Polymers the joining of adjacent long-chain polymers by bonding of short chains along their sides
Free Radical a reactive group on one end of a monomer that initiates the joining of adjacent monomer molecules to form a polymer
Poly (Methyl Methacrylate) a polymer composed of numerous methyl methacrylate monomers linked together into a long chain
Plasticizer liquid added to acrylic resin to soften it and make it more pliable
Long-Term Soft Liner a soft liner that is used in patients who have problems with hard acrylic denture bases; it is expected to last for 1 to 3 years
Short-Term Soft Liner a soft provisional liner used to improve tissue health; also called a tissue conditioner; typically, it lasts from a few days to a few weeks
Acrylic resins are polymers that play an important role in removable prosthetics. The resins are used in the construction of complete and partial dentures, as well as maxillofacial prostheses (used to replace missing oral or facial structures). They can be used to simulate the oral mucosa and the teeth. They are also used to reline these prostheses to improve their fit and to repair them when they break. Even though a commercial dental laboratory fabricates most of the removable prostheses, the allied oral health practitioner needs to be familiar with the materials and their uses, care, and repair. She or he must understand the materials used in prosthetic dentistry to better care for the patient and to assist the dentist. Patients who wear removable prostheses will often ask the dental assistant or hygienist questions that relate to the fit or home care of their prosthesis. This chapter covers the uses of acrylics for construction of complete and partial dentures and for relining, tissue conditioning, and repairing these prostheses. It also covers the use of acrylic denture teeth, porcelain denture teeth, and acrylic repair techniques. The instructions to be given to the patient for the care of these prostheses are presented.
Review of Polymer Formation
Polymers are large, long-chain molecules formed by chemically joining together smaller molecules, called monomers. When two or more different types of monomers join together, the polymer formed from them is called a copolymer. Copolymers are produced to enhance the physical properties of the material. The act of forming polymers is called polymerization.
Cross-Linked Polymers
The polymer chains often have short chains of atoms attached to their sides. When the side chains of adjacent polymers bond together, the polymers are termed cross-linked polymers (see Chapter 6, Figure 6-4). When side chains of adjacent polymers are joined by weak bonds, the polymers are easily manipulated, bent, or stretched. When adjacent polymers are joined by highly charged side chains, the bond is stronger, and the cross-linked polymers are stronger and stiffer.
Polymerization Reactions
There are two types of polymerization: addition and condensation. The reactions are the same as for the impression polymers, addition silicones, and condensation silicones (see Chapter 14).
Addition Polymerization
Addition polymerization is the most common form of polymerization for dental materials. It occurs in three stages: initiation (or induction), propagation, and termination. Monomers have a core unit of two carbon atoms joined by a double bond. One carbon atom has two hydrogen atoms attached, and the other carbon atom has attached to it one hydrogen atom and one reactive group called a free radical. The free radical is made reactive by the chemical reaction of organic peroxide, such as benzoyl peroxide, with an activator or accelerator, such as a tertiary amine, or by heating. The free radical initiates the reaction by opening the bond between the two carbon atoms of the monomer. The broken carbon bond causes the monomer molecule to bond to another monomer. Each linkage leaves a free radical available for further reaction. This process of linking monomer units is termed propagation, and it continues until the monomer units are used up, or until a substance reacts with the free radical to tie it up. When the free radical is tied up or destroyed, the process is terminated. The materials that react by chemical means are called chemical-curing, self-curing, or auto-polymerizing. Materials that use heat to initiate the reaction are called heat-curing polymers. Materials in which the reaction is activated by light are called photo- or light-cured. Whether initiated by chemical means, light, or heat, the polymerization process itself releases heat. The heat must be controlled during the process. If the temperature becomes too great, the monomer will vaporize and produce porosity in the material. Porosity weakens the material, causes it to discolor as stains are absorbed into the pores, and can lead to retention and growth of oral microorganisms.
Condensation Polymerization
Materials formed by a condensation reaction do not have many uses in dentistry. The condensation silicone impression materials are the most commonly known, and even they are not used much today. Typically, more than one type of monomer is used. The reaction itself produces by-products such as water, hydrogen gas, or alcohol that may compromise the physical properties or handling characteristics.
Acrylic Resins (Plastics)
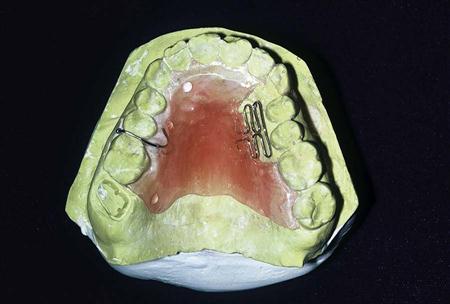
Synthetic polymers used in prosthetic dentistry are called acrylic resins, because they are derived from acrylic acid. Acrylic resin forms when a liquid monomer is mixed with a powder of small polymer beads, and the mixture undergoes polymerization. The polymerized resin is poly (methyl methacrylate) (PMMA). It is composed of numerous methyl methacrylate (MMA) monomer units linked together to form a long-chain polymer. The resins are used for denture bases, denture teeth, reline and repair of prostheses, provisional acrylic partial dentures (flippers or stayplates), tissue conditioners, and custom impression trays. They also have uses for orthodontic retainers and removable tooth movement devices (Figure 16-1), bruxism mouth guards, fluoride and bleaching trays, facings on esthetic crowns, and provisional restorations (see Chapter 17). Specialized acrylic resins are used in esthetic tissue replacement for severe gingival recession and for facial reconstruction due to trauma, surgery, or birth defects. Acrylic resins are especially useful because they can be shaped to any contour and custom-colored to match the shade of the teeth, gingiva, or skin. Acrylic resins used in dentistry are often modified by the addition of plasticizers, rubbers, and fillers to change their physical properties. Plasticizers are liquids added to soften the acrylic plastics. Oily liquids called aromatic esters are often used as plasticizers. Rubbers may be added to increase the impact fracture resistance of the acrylic resin, and fillers are added to strengthen the resin.
Properties
Polymerization Shrinkage
Polymers undergo shrinkage as a result of the polymerization process. Heat-cured acrylic resins shrink about 6% by volume and about 0.2% to 0.5% linearly (from one point on the denture to another).
Dimensional Change
Sources of dimensional change in addition to polymerization shrinkage include water sorption and thermal expansion. A denture base will increase slightly in its overall size when it absorbs water. This expansion may help offset some of the shrinkage that occurs during polymerization. The coefficient of thermal expansion is more than twice that of composite resins (see Chapter 6).
Strength
The strength of the acrylic resins is fairly low, with a compressive strength of approximately 11,000 psi (73.3 MPa) and a tensile strength of 8000 psi (53.3 MPa). By comparison, amalgam has a compressive strength of about 60,000 psi (400 MPa). Acrylics are not very hard materials (Knoop hardness number 16 to 18) and as a consequence are not very wear resistant. Although they have a fairly good resistance to fatigue failure (can be flexed repeatedly before they break), they will break if dropped on the floor or in an empty sink during cleaning. To combat the brittleness and breakage problem, some manufacturers add butadiene-styrene rubber to the MMA to create a high-impact acrylic resin. An example of a high-impact acrylic resin is Lucitone 199 (Dentsply International, York, PA), and it comes in four gingival shades.
Thermal Conductivity
Denture bases do not conduct temperature well. Patients wearing dentures will notice a marked difference when they eat foods such as ice cream or drink hot beverages. Because the denture partially insulates against the temperature of the food or beverage, patients may burn themselves when they attempt to swallow foods that are too hot.
Chemical-Cured Versus Heat-Cured Acrylics
The type of processing has some effect on the properties. Polymerization is never 100% complete, and varying amounts of free monomer may be present in the polymerized material. In general, the chemical-cured acrylic resins are weaker, softer, more porous, and less color stable than the heat-cured acrylic resins. After polymerization, there is more residual monomer in the chemical-cured acrylic (up to 5%), and it initially adversely affects many of the physical properties until the monomer leaches out in minute amounts over several days or weeks. The tissues of some patients react to the residual monomer and become inflamed. Some dimensional change can occur during the first 24 hours in chemical-cured acrylic. For this reason, custom acrylic trays should not be used immediately, but should sit for 12 to 24 hours to allow most of the dimensional change to occur, so the impression will not be distorted. Heat-cured acrylics are harder, stronger, and less porous and have less than 1% residual monomer that leaches out of the surface relatively quickly. Properties of heat-cured acrylic resins are summarized in Table 16-1.
TABLE 16-1
Properties of Heat-Cured Acrylic Resins (PMMA)
| Polymerization shrinkage (by volume) | 6% |
| Polymerization shrinkage (linear) | 0.2%–0.5% |
| Coefficient of thermal expansion | More than twice that of composite |
| Compressive strength | 76 MPa (11,000 psi) |
| Tensile strength | 55 MPa (8000 psi) |
| Hardness (Knoop) | 15–18 kg/mm2 |
| Biocompatibility | Good |
| Thermal conductivity | Poor |
| Wear resistance | Fair |
| Fatigue resistance (to flexing) | Good |
| Impact resistance (to breakage when dropped) | Poor |
Porosity
Porosity in polymerized acrylic resin is characterized by the presence of many small or microscopic voids or pores. Porosity in the acrylic weakens it and makes it prone to collect debris and microorganisms. Denture odor and stains develop more readily. Porosity is a result of loss of monomer or inadequate pressure during processing. The monomer is highly volatile and can evaporate rapidly at room temperature during handling of the mixed powder and liquid. Monomer can vaporize during heat-curing of the resin if the temperature rises too much. Curing under pressure helps keep the monomer from evaporating during polymerization and creates a denser acrylic. When the chemical-cured acrylic is cured in room temperature water and 15 to 20 pounds of air pressure in a pressure pot (Figure 16-2), the resulting acrylic is stronger and has less porosity and shrinkage. A pressure pot is a laboratory device that resembles a pressure cooker. It is a thick-sided metal pot that seals well and can be pressurized by the addition of air through a one-way valve in its lid. Porosity can occur also by the entrapment of air during mixing of the powder and liquid acrylic resin components.
Acrylic Resin for Denture Bases
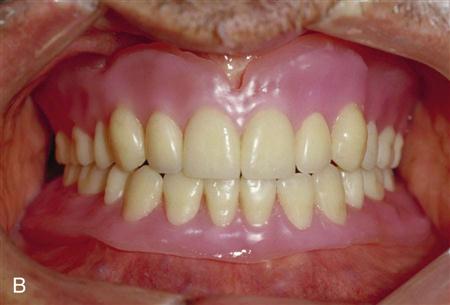
The primary function of the acrylic resin denture base is to retain the artificial teeth in the prosthesis (Figure 16-3). It also serves to distribute forces of mastication over a wide area so as to reduce pressure on the ridges that might contribute to resorption of the underlying bone. Additionally, the denture base replaces missing tissues or rebuilds the contours of tissues lost when the underlying bone resorbs. In complete dentures, the base establishes a seal along the periphery of the denture that aids in retention.
Polymerization Reaction
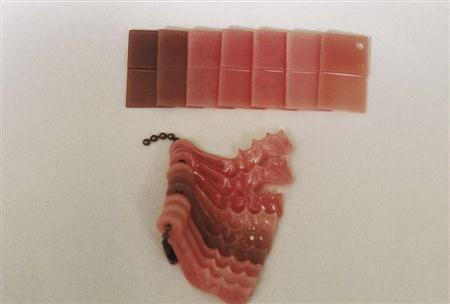
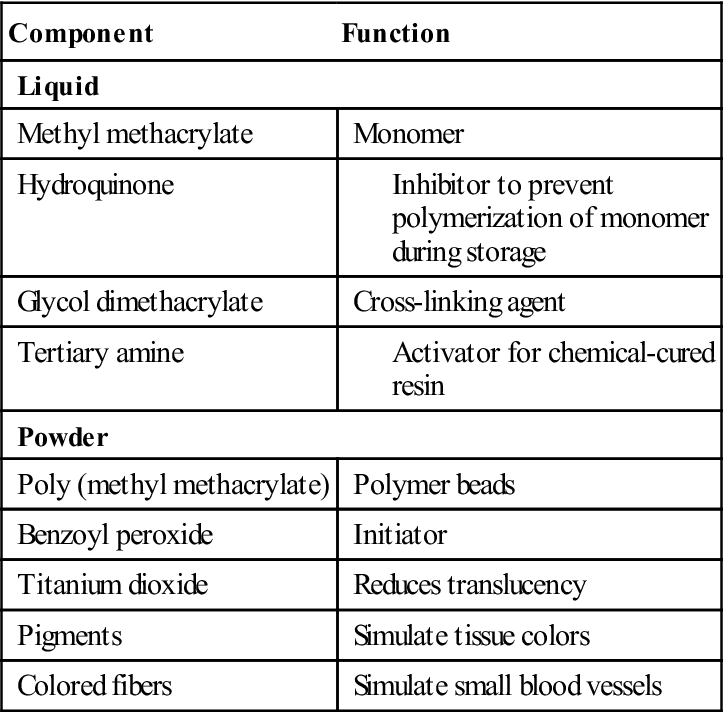
Acrylic resins polymerize by an addition reaction. Acrylic resins are supplied as a powder and a liquid. The powder is composed mostly of small beads of PMMA and benzoyl peroxide (the initiator). Inorganic pigments are added to give the acrylic resin colors resembling oral mucosa, and titanium dioxide is added to keep the acrylic from being too transparent. Small, colored fibers may be added to simulate small blood vessels. Several shades of acrylic are available, so that the clinician can attempt to match the variations of racial pigmentation that can occur in the gingiva and mucosa of patients (Figure 16-4). The liquid contains MMA, hydroquinone as an inhibitor or preservative to prevent polymerization of the MMA during storage, and glycol dimethacrylate as a cross-linking agent. Cross-linking of the acrylic polymer chains helps prevent surface cracks, and it improves resistance to structural fatigue that can lead to fracture. The liquid is supplied in dark brown bottles to prevent ultraviolet light from initiating polymerization during storage. When the powder and the liquid are mixed, chemical- and heat-cured materials go through a similar reaction, except that chemical-cured materials have a tertiary amine in the liquid as an activator, whereas heat-cured materials do not (Table 16-2).
TABLE 16-2
Ingredients of Acrylic Resin and Their Function
< ?comst?>
< ?comen?>< ?comst1?>
< ?comst1?>
< ?comen1?>
The first stage seen when the powder (polymer) and the liquid (monomer) are mixed is called the sandy stage, because the mixture looks grainy, similar to sand and water, and has a runny consistency. The second stage occurs when the powder particles absorb the liquid into their surface. It is called the stringy stage, because the mixture is stringy when handled and is thicker in consistency. In the next stage, called the dough stage, more of the powder goes into solution and the mixture changes from stringy to doughy and is more easily manipulated. In the final stage, called the rubber stage, the mixture has a rubbery consistency that can no longer be manipulated for forming the denture base.
Chemical-Cured Resins
Polymerization of chemical-cured acrylic resins is set into action when the tertiary amine in the liquid activates the benzoyl peroxide in the powder to produce free radicals. The hydroquinone initially inhibits the reaction from progressing by destroying the free radicals. This inhibition increases the working time so that the materials can be manipulated for a reasonable period of time, usually while the material progresses from the stringy stage to the dough stage. As the hydroquinone is depleted, the reaction proceeds more rapidly and goes from the dough stage to the rubber stage. The reaction is exothermic (releases heat) and goes from warm to quite hot. When the reaction is complete, the material is hard and stiff.
Pour Technique
Some chemical-cured materials can be mixed to a thin, fluid consistency and poured into a mold and cured under pressure.
Heat-Cured Resins
The most common method for processing denture bases uses heat and pressure during polymerization of the acrylic resin. Heat-cured acrylic resins go through initial stages of polymerization that are similar to those of chemical-cured resins. After the dentist has tried in the denture teeth set in wax and the patient approves the appearance, the denture is returned to the laboratory and the technician places the cast of the edentulous arch, along with the denture setup (teeth mounted in wax on a record base), in a specially designed processing flask. The flask separates in the middle into two sections. The denture setup mounted on the cast is invested in a plaster investing material in the flask in such a manner that the flask can be opened in the middle to allow removal of the wax and record base after heating in a water bath. The teeth are held />
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses