Skin and Composite Grafts
Skin Anatomy and Physiology
The epidermis is attached to the dermis by a basement membrane zone that extends from the epidermis to pilosebaceous units and sweat ducts located in the dermis. Each pilosebaceous unit contains sebaceous glands, a hair shaft and follicle with associated arrector pili muscle, and a sensory end organ. Epithelialization of partial-thickness skin wounds occurs from wound edges and the basement membrane zone around the hair follicles, sebaceous glands, and sweat ducts.1
The dermis is divided into a thin papillary and a thicker reticular dermis. The overall thickness of the dermis is variable, depending on its location. Eyelid skin has the thinnest dermis, measuring less than 1 mm thick. Dermal thickness measures 1.5 mm on the temple, 2.5 mm on the scalp, and more than 4 mm on the back. The dermis is thin at birth, increases in thickness until the fourth or fifth decade, and then decreases with further aging. On average, men have a thicker dermis than women do.1
Cutaneous blood flow is directed toward the more metabolically active epidermis through dermal papillae, hair papillae, and adnexal structures. Two vascular plexuses connected by communicating vessels are present in the reticular dermis. A deep plexus lies at the junction of dermis and fat; the superficial plexus gives rise to a rich capillary loop system in the superficial dermal papillae. This system provides nutrients to the epidermis through diffusion.2
Skin Grafts
On occasion, superficial facial defects are best addressed by repair with a skin or composite graft. Very young patients with tight facial skin presenting with large cutaneous defects are appropriate candidates for reconstruction with a full-thickness skin graft. Use of a skin graft is usually reserved for those cases that cannot reasonably be reconstructed with a local flap because of the size of the defect. The skin graft obviates the need for additional scars on the face or the use of a distant or microsurgical flap. Unfortunately, full-thickness skin grafts used in such cases frequently have a “patch” appearance, with discrepancies of color and texture between native and grafted skin (Fig. 15-1). Color and texture discrepancies between facial skin and skin grafts may also be a problem in elderly patients (Fig. 15-2). However, because of the tendency for elderly patients to have thin skin, grafts used to repair facial defects are more likely to display a better color and textural match with native facial skin than when similar grafts are used in younger patients (Fig. 15-3). Postoperative dermabrasion assists in minimizing color and texture discrepancies between skin grafts and facial skin.
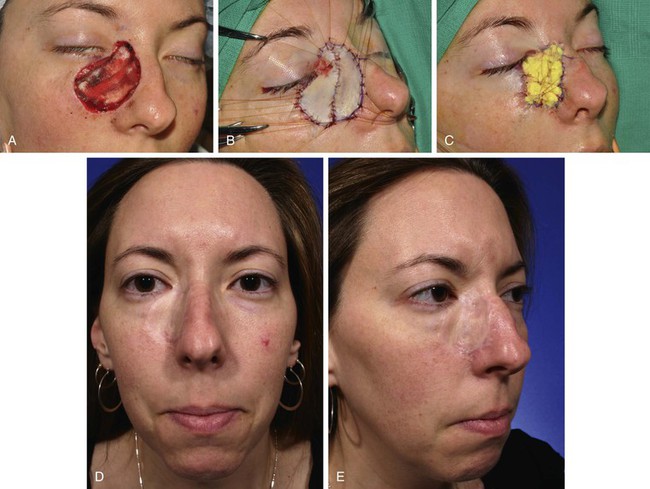
FIGURE 15-1 A, A 4.5 × 4.2-cm skin defect after micrographic excision of basal cell carcinoma in a 34-year-old woman. B, C, Repair with full-thickness skin graft from supraclavicular fossa. D, E, Postoperative views at 11 months. No adjunctive procedures performed. Graft has poor color and textural match with facial skin. (Courtesy Shan R. Baker, MD.)
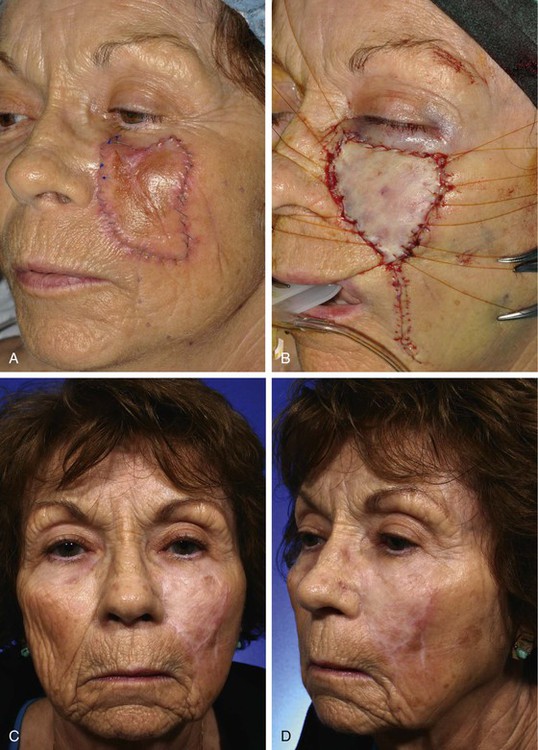
FIGURE 15-2 A, A 5 × 4-cm melanoma in situ. Suture line marks planned excision. B, Melanoma excised. Resulting wound partially closed primarily. Remaining wound covered with 3.5 × 4-cm full-thickness skin graft. C, D, Postoperative views at 1 year. Z-plasties performed at superior border of graft. (Courtesy Shan R. Baker, MD.)
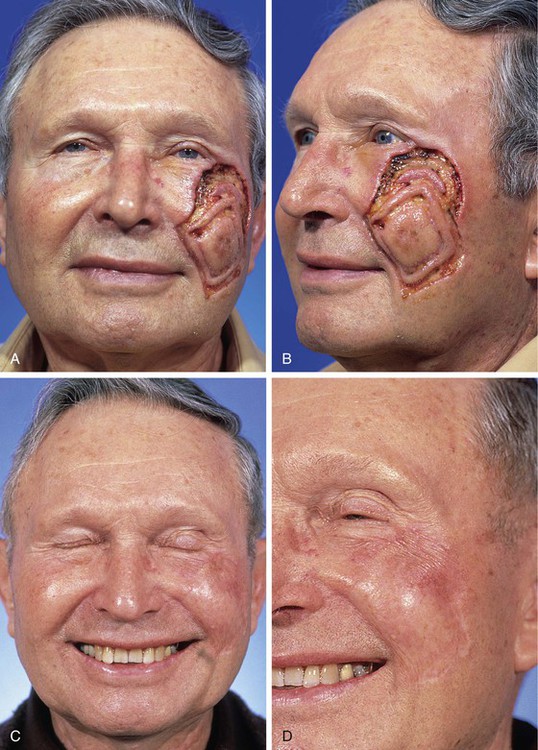
FIGURE 15-3 A, B, Lentigo maligna of cheek outlined by linear incisions from attempts to obtain tumor-free margins before complete resection (square technique; see Chapter 8). Resected area (7 × 6 cm) was reconstructed with full-thickness skin graft harvested from supraclavicular fossa. C, D, Postoperative views at 10 months. Skin graft provided an excellent color and textural match with native facial skin. No adjunctive procedures performed. (Courtesy Shan R. Baker, MD.)
Contact between the skin graft and recipient site is essential. A bolster dressing is helpful to prevent fluid collections beneath the graft postoperatively (Fig. 15-4). Bolsters also prevent shearing forces from disrupting vascular connections between graft and wound bed. Systemic illnesses that may compromise graft survival include collagen vascular diseases, hematologic disorders, diabetes, nutritional deficiencies, and hypoxemia.3 Use of tobacco products is also detrimental to the survival of skin grafts.
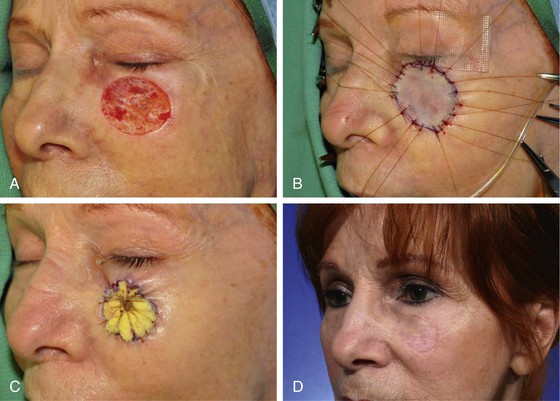
FIGURE 15-4 A, A 3 × 2.7-cm skin defect of cheek after micrographic excision of squamous cell carcinoma. B, Defect repaired with full-thickness skin graft from supraclavicular fossa. C, Gauze bolster dressing secures graft in place for 5 days. D, Postoperative view at 1 year. (Courtesy Shan R. Baker, MD.)
Recipient site conditions that are not favorable to skin graft survival include irradiated tissue; scar; exposed bone, cartilage, or tendon; and bleeding wounds. Grafts placed over avascular defects smaller than 1 cm2 may survive by nutritional support through the wound edges; however, grafting over larger avascular wounds is unlikely to succeed.3 Skin graft survival on bone is enhanced if a thin layer of bone is removed with a diamond fraise bur until punctuate bleeding is achieved. On the skull, holes can be made in the outer bone table to allow communication with the inner diploë. Large areas of exposed bone, cartilage, or tendon may require coverage with a muscle or fascial flap before grafting. Another technique that has proved successful in skin grafting auricular cartilage devoid of perichondrium is to create windows through the cartilage, exposing perichondrium and subcutaneous tissue on the opposing surface of the ear. The windows are made as large as possible through the cartilage in areas that can be resected without jeopardizing the structural integrity of the ear. The windows provide a port for vascular ingrowth to revascularize the graft (Fig. 15-5). Only very thin full-thickness skin grafts should be used for covering auricular cartilage so that the graft will not obscure the complex and delicate topography of the cartilage. Thin skin grafts are more likely to survive compared with thick full-thickness skin grafts, given the limited contact with a vascular source for nourishment.
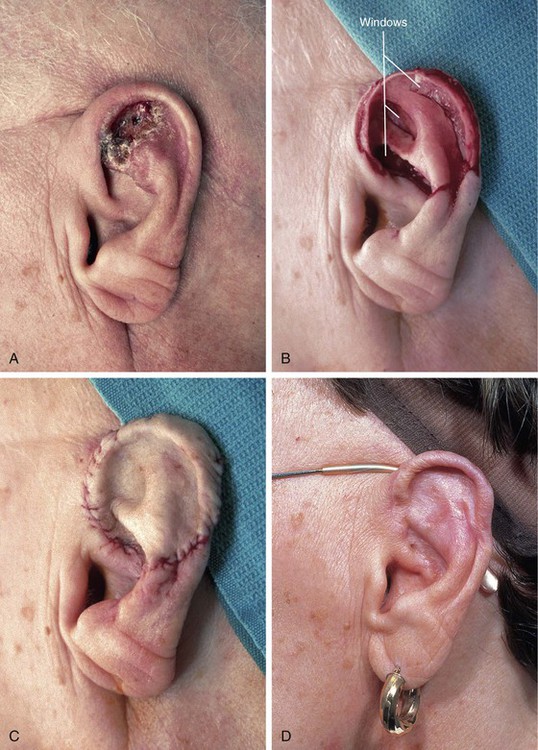
FIGURE 15-5 A, Basal cell carcinoma of auricle. B, After tumor resection, windows made through cartilage for portals of ingrowth of blood vessels to nourish skin graft. C, Thin full-thickness skin graft in place. D, Postoperative view at 6 months. No adjunctive procedures performed. (Courtesy Shan R. Baker, MD.)
For deeper wounds, skin grafting may be delayed until granulation tissue has filled the wound bed (2-3 weeks). Any epithelium on the surface of the granulation tissue is removed before grafting, and the tissue is crosshatched so that myofibrils are released. Granulating wounds normally contain bacteria. Bacterial counts greater than 105 organisms per gram of tissue often lead to graft loss.4 When delayed grafting is planned, the patient is prescribed a 10-day course of an antistaphylococcal antibiotic to begin 3 days before skin grafting. When necessary, wound culture specimens are obtained to direct antibiotic selection.
Defect Preparation
The aesthetic facial regions and their individual aesthetic units are based on variations in skin thickness and texture as well as on variations in contour created by the underlying facial framework. Optimal repair of a defect may require repositioning of skin and soft tissue within an involved aesthetic unit, thereby allowing eventual scars to lie within zones of transition between adjacent units. In addition, small defects may be enlarged to facilitate resurfacing of an entire aesthetic unit by a single repair method.
Full-Thickness Skin Grafts
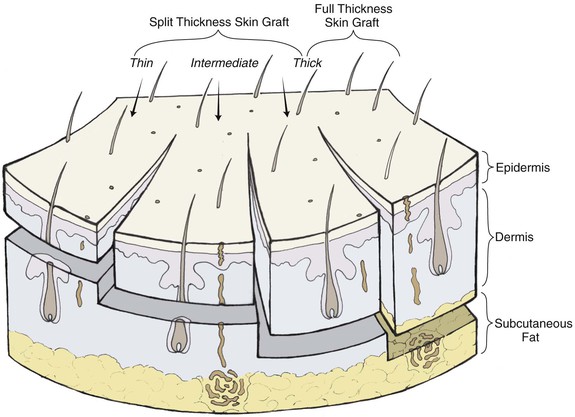
Full-thickness skin grafts consist of epidermis and full-thickness dermis (Fig. 15-6). They resist contraction, have texture and pigmentation similar to normal skin, and require a well-vascularized, uncontaminated recipient site for survival. Full-thickness grafts survive initially by diffusion of nutrition from fluid at the recipient site, a process known as plasma imbibition. Vascular inosculation occurs during the first 24 to 48 hours. After 48 to 72 hours, capillaries in the recipient site begin to grow into the graft to provide new circulation. By 3 to 5 days, a new blood supply has been established. Initially, full-thickness skin grafts appear blanched; however, during 3 to 7 days, a pink color develops, signaling neovascularization. After 4 to 6 weeks, the pink color begins to fade, and the graft will often remain lighter than the surrounding skin, especially in dark-skinned individuals.
Compared with split-thickness skin grafts, full-thickness skin grafts have the advantage of better color and texture match, fewer contour irregularities, no need for special equipment to harvest the graft, and easier donor site wound care. The disadvantages include reduced survival rate for larger grafts and longer healing time.3
Full-thickness skin grafts are well suited for defects located in thin-skinned areas of the face (eyelids, nasal sidewall, columella) and for defects involving concavities (temple, medial canthus) (Figs. 15-7 and 15-8). Full-thickness skin grafts used to repair defects of the face in regions of thicker skin tend to heal with a contour depression and noticeable textural discrepancies between graft and adjacent skin. This is because the skin in these areas contains more sebaceous glands and has a thicker dermis than the graft. However, contour discrepancies between skin grafts and adjacent nasal skin frequently improve with time as scar tissue forms beneath the graft (Figs. 15-9 and 15-10). If a skin graft has resulted in a persistent contour depression, the appearance of the graft may be improved by placement of a dermal fat graft beneath the skin graft after it has healed (Fig. 15-11). Meyers et al5 described the use of dermal grafts at the time of skin graft placement to help prevent contour depression after repair of deeper defects. Their technique involves the placement of dermal tissue in linear strips within the wound bed, leaving adequate exposure of the wound bed to provide nourishment to an overlying skin graft.5 The author’s preference is to perform a delayed dermal fat graft 3 months postoperatively if a significant contour deformity exists.
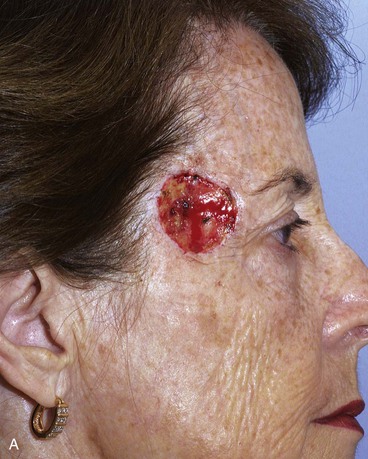
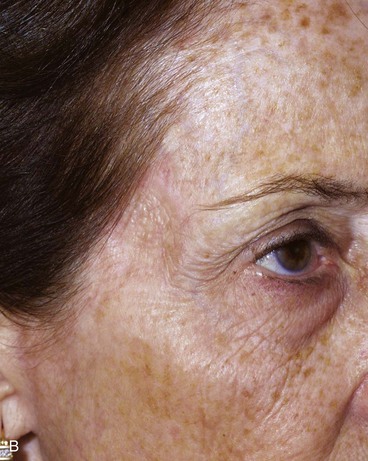
FIGURE 15-7 A, A 2 × 2-cm skin defect of temple. B, Six months after repair with preauricular full-thickness skin graft. No adjunctive procedures performed.
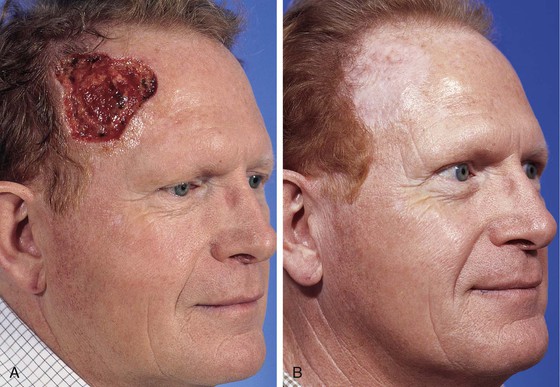
FIGURE 15-8 A, A 6 × 6-cm skin defect of temple after micrographic surgery for basal cell carcinoma. B, Five months after repair with full-thickness skin graft from supraclavicular fossa. No adjunctive procedures performed. (Courtesy Shan R. Baker, MD.)
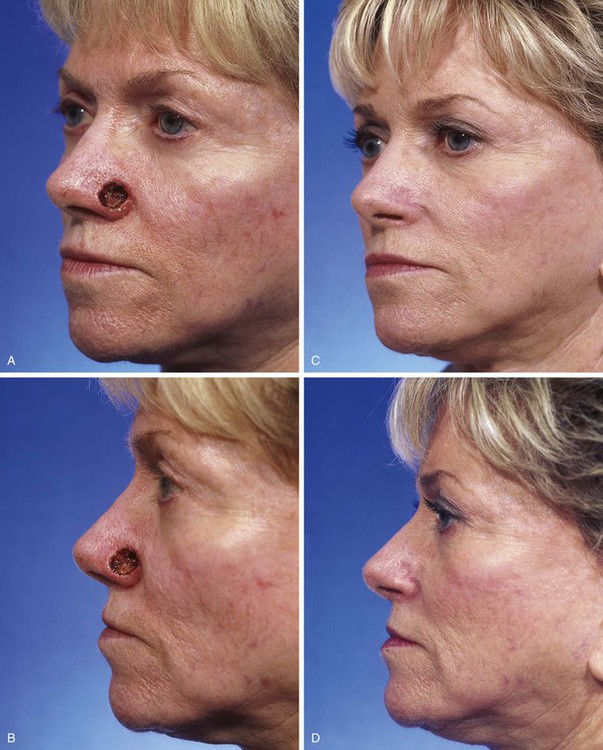
FIGURE 15-9 A, B, A 1.5 × 1-cm-deep defect of ala repaired with full-thickness skin graft from supraclavicular fossa. C, D, Postoperative views at 7 months. Scar tissue beneath graft assists with filling depth of wound, preventing contour discrepancy between graft and adjacent nasal skin. Graft was dermabraded. (Courtesy Shan R. Baker, MD.)
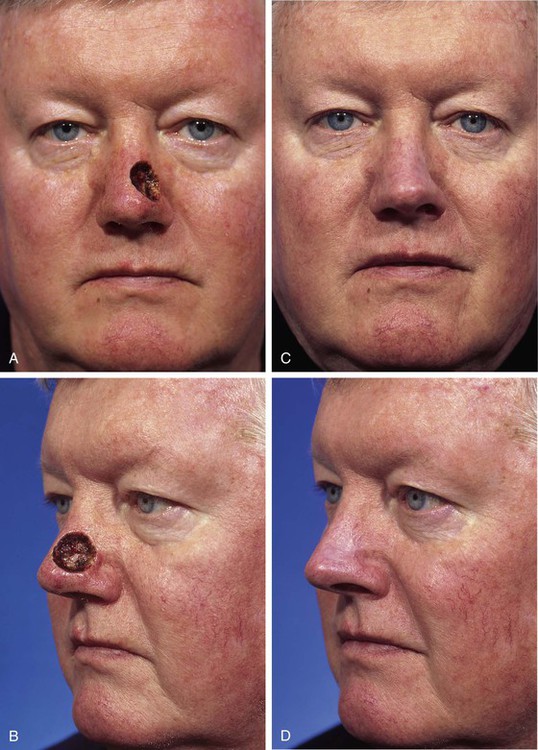
FIGURE 15-10 A, B, A 2 × 2-cm-deep skin and soft tissue defect of nasal tip and sidewall. Repair with paramedian forehead flap recommended but patient declined. Defect repaired with full-thickness skin graft from supraclavicular fossa. C, D, Postoperative result at 5 months. No adjunctive surgery performed. No contour deformity occurred secondary to scar tissue deposition beneath skin graft. (Courtesy Shan R. Baker, MD.)
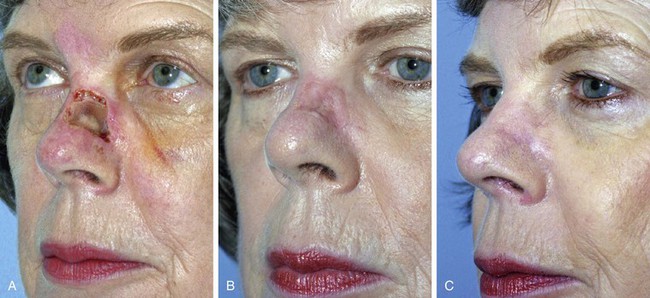
FIGURE 15-11 A, A 2 × 1.5-cm skin defect of nasal sidewall with exposed periosteum. Defect repaired with full-thickness skin graft. B, Depressed contour of graft 3 months later. C, One year after staged dermal fat graft placed beneath skin graft and subsequent dermabrasion of skin graft.
There is a wide variation of skin thickness among individuals, and the overall thickness of the facial skin is an important preoperative consideration. For similar defects, a skin graft may provide a perfect match in terms of thickness for one person and a poor match for another. There are individuals who have thin skin covering the entire face. These individuals are often fair-skinned elderly women, and full-thickness skin grafts may be used in these patients for superficial cutaneous defects anywhere on the face without concern for color or textural discrepancies between graft and facial skin (Fig. 15-12). The only exception to this rule is in the area of the margins of the nostril, eyelid, and vermilion; scar contraction after skin grafting will likely distort the border of these structures.
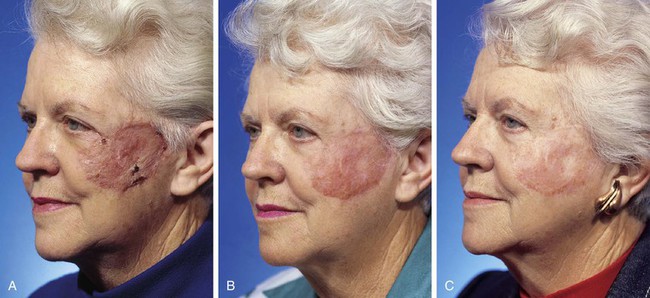
FIGURE 15-12 A, One month after full-thickness skin grafting of cheek. Graft harvested from supraclavicular fossa. B, Three months later. Graft has developed postinflammatory hyperpigmentation. C, Postoperative view at 10 months. Hyperpigmentation has resolved. There are fewer skin color and textural discrepancies between graft and native skin in fair-skinned elderly patients. (Courtesy Shan R. Baker, MD.)
A number of donor sites for skin grafts are available in most individuals, depending on the location and size of the facial defect. Donor sites include upper eyelid, forehead, melolabial fold, preauricular, postauricular, and supraclavicular areas (Fig. 15-13). In selecting a donor site, the thickness of the skin surrounding the recipient site is assessed and donor skin is selected accordingly. Regions of the face with thicker skin include the forehead, medial cheek, caudal nose, and chin. Skin defects in these areas can be repaired with grafts from contralateral facial donor sites and periauricular or supraclavicular areas (Fig. 15-14). Regions of the face with thinner skin, such as the eyelids and cephalic two-thirds of the nose, are best repaired with skin grafts obtained from the eyelid or periauricular area that are thinned appropriately. Skin grafts harvested from the postauricular area are often preferred in men because they are hairless. Men tend to have shorter hair than women do, causing the postauricular skin to more likely manifest solar aging, which provides an improved skin color match with the facial skin (Fig. 15-15). In contrast, preauricular skin is often the prefer/>
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses