14 Principles of Biocompatibility
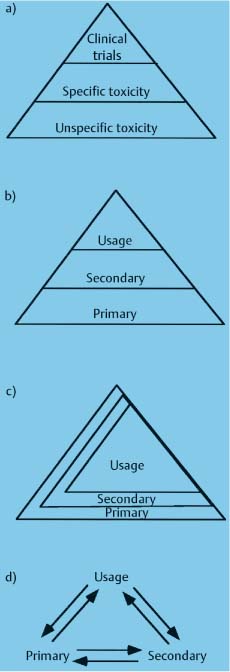
Schemes for testing biocompatibility. (a) The oldest scheme used “unspecific” toxicity tests first, followed by “specific“ toxicity tests, and then by clinical trials. Only materials that passed one level were tested further. Unspecific tests were those that were not directly relevant to the use of the material. (b) This scheme employs primary, secondary, and usage tests, and it is still commonly employed today. The scheme differs from (a), because it emphasizes many cellular reactions in addition to toxicity. As in (a), each level of the test screens for the tests above it. Primary tests measure basic biological properties such as toxicity or mutagenicity of the material. Secondary tests assess more advanced properties such as allergenicity. Usage tests are equivalent to clinical trials. (c and d) Newer schemes for biocompatibility testing that recognize the need to use several types of tests together and that the evaluation of biocompatiblity of a material is an ongoing process
Introduction: Relevance of Biocompatibility to Dentists
Compliance with Regulatory Issues
Definition of Biocompatibility: Concepts and Misconceptions
Accepted Definition and Key Points
Relevance to Dental and Orthodontic Practitioners
Measurement of Biocompatibility
Types of Biocompatibility Tests
Correlation Among Types of Tests
Using Different Tests Together
Relevance to the Dental and Orthodontic Practitioner
Standards and Biocompatibility Testing
Relevance to the Dental and Orthodontic Practitioner
Key Factors in Assessing Biocompatibility
What is Released from the Resin-Based Material?
What are the Effects of the Released Components of Resins?
Relevance to the Dental and Orthodontic Practitioner
Introduction: Relevance of Biocompatibility to Dentists
Biocompatibility is a complex and rapidly evolving research area that may seem beyond the purview of practicing dentists. However, bio-compatibility issues should be of concern to every practitioner because these issues have profound ethical, social, technical, and legal implications for dental practice. The practitioner’s potential concerns about biocompatibility can be organized into four areas: safety issues for the patient, safety issues for the dental team, compliance issues, and liability.
Safety of the Patient
Clearly, one of the primary concerns of any dental practitioner is to avoid harming the patient. This concern is especially important in orthodontic practices where many patients are children or young adults. Throughout the years there is evidence that, while adverse reactions to dental materials are not common, they can occur for many types of materials used in orthodontics, including alloys, resins, and cements. A national registry in Norway, where there are 4.3 million people and 3800 dentists, reported 674 adverse reactions from 1993 to 1997. These adverse events involved all classes of dental materials and occurred locally and systemically. A relatively rare frequency of problems (1:2600) has been reported for dental casting alloys, including the nickel-based alloys used in orthodontics. Yet most researchers agree that, because many adverse reactions may be unreported, existing reports may not be accurate, and that better documentation of the extent of these reactions is needed.
Two biocompatibility-patient safety issues have been prominent in orthodontics in recent years. The first is the hypersensitivity of the patient to dental biomaterials. Classically, this concern has focused on nickel allergy. The incidence of nickel allergy in the general population is somewhere between 10% and 20%, being far more common in females than males. The frequency with which nickel elicits adverse responses in sensitive patients through oral exposure is controversial, but rarely can be spectacular. Chapter 15 deals with these issues in depth. There is also growing concern about hypersensitivity of the patient to latex and other resin-based materials. Although not common, patients can suffer severe or even fatal allergic reactions to these materials. There have been reports of a growing incidence of contact sensitivity to a variety of substances, including dental materials, in children. One such report found that 49% of children had sensitivity to some type of material or food. Since orthodontists treat many children, these types of reports deserve scrutiny.
The second major biocompatibility issue that has faced orthodontists in recent years is the question of the estrogenicity of dental resins, particularly those containing the chemical bisphenol A, one of the two components that react to form Bis-GMA (Chapter 9). Estrogenicity is the ability of chemicals from the environment, called xenoestrogens, to mimic the hormone estrogen in the body of the exposed person. Because natural estrogens have many powerful and widespread effects on growth and development, xenoestrogens are a concern for all animal life in our environment. A single article published in 1996 claimed that dental sealants released sufficient amounts of the xenoestrogen bisphenol A to be a plausible threat to children. Although unsubstantiated by other reports, these issues are alarming to dental patients and capture significant coverage in the media. The issue of bisphenol A in dentistry is covered more extensively at the end of this chapter.
Thus, there is evidence that the biomaterials used by dental practitioners can pose some risk to patients. It is the practitioner’s problem to decide whether this evidence has merit and to assess the risks of these issues in his or her own practice.
Safety of the Dental Team
The area of biocompatibility of materials is also relevant to the practitioner from the standpoint of the health of the dental team. In many cases, the risk of adverse effects of biomaterials is much higher for the dental team than for the patient because of the chronic exposure of the dental team and the manipulation of materials when they are being placed, setting, or being removed. The classic example of this problem is with dental amalgam, since the release of mercury vapor from amalgam during placement or removal is substantially higher than after it has set in the cavity preparation. However, these types of issues are also relevant to the orthodontist.
The primary risk for the dental team in orthodontics appears to be contact with latex-based and resin-based materials. These adverse effects can be from cumulative irritation or from allergenic responses. Ironically, not only do gloves not protect against contact with some of these materials (which are capable of moving through gloves), but the gloves themselves are a source of the problem. One study in Sweden reported that 15% of dentists (vs. 9% in the general population) had itching on the hands in response to gloves, particularly latex gloves. The mechanisms by which these materials cause problems are not known, but there is evidence that some resin components such as HEMA (hydroxyethyl methacrylate), TEGDMA (triethylene glycol dimethacrylate) and camphoroquinone (Chapter 9) are capable of activating immune cells directly. In any case, the reactions to many types of dental materials can be severe, career-threatening, and even life-threatening in rare cases to dental practitioners and auxiliaries. It is therefore in the best interest of the practitioner to understand dental biocompatibility issues.
Compliance with Regulatory Issues
Practitioners are affected by biocompatibility issues because these issues are closely linked to regulatory issues that govern dental practice. An example of this link is with dental amalgam. Because of the biological concerns about mercury, regulators have considered monitoring and restricting the amount of mercury in wastewater from dental practices. This debate has spurred considerable research in methods to eliminate particulate and elemental mercury from dental waste. Most dental practitioners in the United States are keenly aware of the many regulations imposed by the Occupational Health and Safety Administration (OHSA) of the federal government on dental practice. These regulations are, for the most part, designed to minimize risks to dental personnel from agents in dental restorative materials and devices. Thus, biocompatibility concerns directly influence regulatory policy.
One current regulatory issue with its origins in biocompatibility is hypersensitivity to latex. Latex rubber and its associated proteins are capable of causing severe and sometimes life-threatening allergic reactions in patients. Although the severest reactions are apparently rare, their occurrence has fostered formation of groups opposed to the use of latex in health care. In turn, several U.S. state legislatures have debated but not passed bans on latex gloves in dental and medical practice. As reactions to latex products become more common and better documented, such regulatory pressures are certain to persist.
Liability
Biocompatibility issues also influence liability issues, which affect dental practitioners. Because dental materials can affect the well-being of patients or dental auxiliaries, the practitioner assumes a legal risk when using these materials. The frequency of litigation as a result of biomaterials causing harm to patients is unclear but is probably low. Nevertheless, when these problems occur, they are at best emotionally and financially stressful, and at worst devastating to the practitioner. The litigious potential of these issues is enhanced by the facts that orthodontic practices treat many children and that many staff are women of child-bearing age. Examples of such issues in orthodontics include loss of enamel from enamel-bracket debonding, systemic allergy to nickel-containing orthodontic wires or appliances, or development in a staff member of a disabling hypersensitivity to an orthodontic resin material. Thus, biocompatibility issues are important to the orthodontic practitioner from a legal standpoint.
Definition of Biocompatibility: Concepts and Misconceptions
Accepted Definition and Key Points
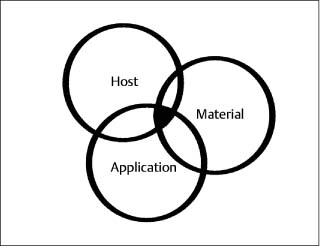
Given the importance of biocompatibility issues to dental practitioners, it is surprising how few understand what biocompatibility really is and what its consequences are. One widely accepted definition of biocompatibility is “the ability of a material to elicit an appropriate biological response in a given application.” If examined closely, this definition implies an interaction between a host, a material, and an expected function of the material (Fig.14.1). All three factors must be considered and be in harmony before the material can be considered biocompatible. A discussion of several key points will reinforce this idea.
The first key point to understand about bio-compatibility is that there are no truly inert biomaterials. When a material is placed into living tissue, interactions with the complex biological systems around it will occur, and those interactions will result in some sort of biological response. The interactions that occur will depend upon the material, the host, and what forces and conditions are imposed on the material (its function). Regardless, the material will affect the host, and the host will affect the material. The term “inert” applied to a biomaterial implies an absence of such interactions. Most scientists agree today that no material is truly inert in the body.
A second key point about the definition of biocompatibility is that it is a dynamic, ongoing process, not a static one. If one has a dental implant that is fully osseointegrated into bone today, does that mean that it will remain osseointegrated over time? The response of the body to a material can change over time (i.e., it is dynamic), because the body may change through disease or aging; the material may change through corrosion or fatigue, or the loads placed on the material may change through changes in the occlusion. Any of these changes may alter the conditions that initially promoted an appropriate and desired biological response. The interactions between material, host, and function continue over time; therefore, the biological response to a material is a dynamic, ongoing process.
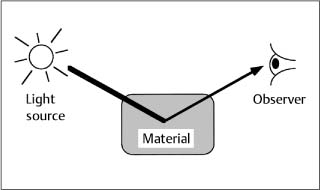
A third key point about the definition of biocompatibility is that it is not a property only of a material, but of a material interacting with its environment. Consider an example with dental and orthopedic implants. A properly placed CP (commercially pure) titanium implant or a plasma-sprayed hydroxyapatite-coated (HA-coated) Ti-6Al-4V implant will osseointegrate with mandibular bone over time. According to currently accepted guidelines, this means that the interface with bone will be located within approximately 10 nm of the implant, without intervening fibrous tissue. If a cobalt-chromium alloy is placed in the same dental implant situation – same host, same placement technique, same load – no osseointegration will occur. Conversely, when Ti-6Al-4V is used as an orthopedic implant alloy for hip arthroplasty, the femoral head (ball) portion of the implant causes wear of the acetabulum (socket), which is typically fabricated from ultrahigh-molecular-weight polyethylene (UHMWPE). The small particles of UHMWPE cause irritation locally and at remote sites because of movement of the debris with circulation of the blood; ultimately failure of the implant occurs. Yet the much harder cobalt-chromium alloy will do well in this orthopedic application. Acetabular cups fabricated from zirconia have been used with the Ti-6Al-4V alloy (which may be HA-coated for more rapid osseointegration), but the zirconia sockets have much greater weight and cause wear of the titanium alloy. Thus, it is not always appropriate to label the Ti-6Al-4V alloy as “biocompatible” and the cobalt-chromium alloy as “incompatible” because this classification depends upon the interaction of the material with its environment. One cannot define the biocompatibility of a material without defining the location and function of the material. In this sense, biocompatibility is like color (Fig.14.2). We often ascribe color to a material, but color is a property not only of the material but also of the interaction of the material with light. Without the light interaction there is no color. The color of the material depends upon the light source, how the light interacts with the material, and the visual response of the observer.
Fig. 14.1 Diagram illustrating the interactions between host, material, and clinical application of the material that are important in biocompatibility
Relevance to Dental and Orthodontic Practitioners
The complexities mentioned in the previous paragraphs may leave the practitioner wondering about the relevance of the definition of bio-compatibility to dental practice. Yet there are profound consequences of this definition for orthodontic practitioners. For example, the practitioner must always consider the health and habits of the patient when assessing the biological response to materials. Is the patient diabetic? Does the patient smoke? If so, the response of the gingivae to metallic bands may be different. Does the patient ingest many acidic drinks? The corrosion properties of the archwires may be different (Chapter 4). The practitioner must consider the particular clinical application and patient, and not assume that, because a certain alloy is biologically acceptable as an archwire, it will be acceptable as a bracket or a band or a spring. Resin materials that are biologically acceptable as removable appliances may or may not be acceptable as resin-based cements. Finally, the practitioner must monitor the patient over time. A patient who is not allergic to nickel today might become allergic in the future.
Fig. 14.2 The color of a material depends upon the interaction of the material with light and the observer’s interpretation of the affected light. Thus, color is a property of a material interacting with its environment. Biocompatibility is also a property of a material interacting with its environment
Measurement of Biocompatibility
Although materials have been used in the body for thousands of years, only recently have the public and various governmental agencies required some assurance that such materials are safe and effective. These regulatory pressures have resulted from many sources, including the movement toward the ethical treatment of patients, an increased awareness of patients about the risks involved in health care, and concerns of health practitioners about litigation by patients. Unfortunately, assessment of the bio-compatibility of a material is a complicated matter that involves several sophisticated types of biological tests, tests of physical properties such as corrosion, and risk-benefit analysis. The complexity in assessing the biocompatibility of materials is often bewildering and frustrating to the practitioner and the public. However, in spite of its complexity, biocompatibility testing of dental materials is the only means currently available to try to assure the safety of the patient and dental team.
Types of Biocompatibility Tests
Biocompatibility is measured using three types of biological tests: in vitro tests, animal tests, and usage tests. Each of these tests is described in detail in the following paragraphs. Although it is unlikely that the practicing orthodontist will need to evaluate results of these tests directly, it is important that he or she understands how materials are approved for use, since ultimately it is the practitioner who must assume the direct legal risks of using materials in the patient. The following paragraphs describe the advantages and disadvantages of each type of biological test, so that the practitioner will have a foundation for understanding the issues that often surround the biocompatibility of materials (Table 14.1).
In vitro biocompatibility tests are performed in a test tube, cell-culture dish, or otherwise outside of a living organism. These tests are quite diverse, but generally place cells or bacteria in contact with a material. For example, a strain of bacteria may be used to assess the ability of a material to cause mutations (the so-called Ames test) or a strai/>
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses