12 Impression Materials
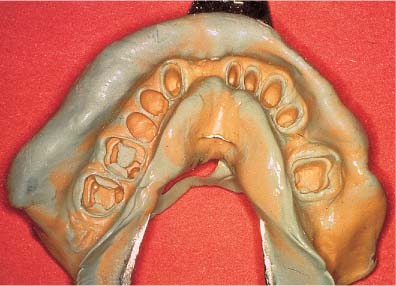
An impression obtained with a polyvinylsiloxane impression material
Classes of Impression Materials
Thermoplastic Bite Impression Materials
Properties of Impression Materials and Relationships to Clinical Use
Properties Before Insertion into the Patient’s Mouth
Properties While in the Patient’s Mouth
Properties During Removal from the Patient’s Mouth
Properties After Removal from the Patient’s Mouth
Introduction
A dental impression material is used to make a mold that has the negative dimensions of the surfaces of a patient’s oral structures. The purpose of the impression is the formation or establishment in the ensuing “positive” model (typically prepared from a dental gypsum product) of the proper physical dimensions, shapes, and spatial relationships of these structures. Most commonly, an impression material is prepared in a low-viscosity state, placed and formed against the oral structures before a significant increase in the viscosity of the material occurs, then removed from the patient’s mouth and used to form the model or to relate models of opposing arches. This increase in viscosity, referred to as setting, can be due to the physical change of cooling or to a chemical reaction. The combination of properties before, during, and after setting determines the suitability of the impression material for each clinical situation.
Materials used to make an impression are usually polymers (e.g., alginate hydrocolloid and polyvinylsiloxane), although some brittle ceramic materials (e.g., dental plaster and zinc oxide-eugenol) may also be used for limited applications. The properties of polymers are dependent upon many factors, but the major factors include the nature of the molecular components and the manner in which these components are bound to one another (Chapter 1). Since changes in molecular binding occur during a setting process, changes in properties accompany setting that are important for successful use of the impression material in the clinic and laboratory. The approach of this chapter is to present some fundamental physical and chemical characteristics of impression materials that are potentially useful to the orthodontist, and then to present the important clinical properties for the ideal impression material with comparisons to available impression materials.
Classes of Impression Materials
The two general classes of impression materials for use in orthodontics consist of those materials that can experience only minimal elastic deformation after setting and those that, after setting, can undergo the large elastic deformations required to remove the impression material over the crowns of all teeth. However, this latter class, which comprises polymeric materials, can demonstrate significant viscous deformation and is more completely described as viscoelastic (Chapter 1). Handling of these materials in a manner that minimizes viscous deformation after setting will promote their successful use clinically.
Thermoplastic Bite Impression Materials
In general, a desirable property of dental impression materials is sufficient elasticity after setting, as discussed more completely below. For example, adequate registrations of the relationship of opposing arches can be removed from the occlusal surfaces of the teeth with minimal deformation. Consequently, bite impression materials require only sufficient elasticity to withstand separation from the occlusal surfaces of teeth. These materials do not require the ability to recover elastically from the large deformations that occur during withdrawal past undercuts on teeth. Since a thermoplastic polymer transforms from a lowviscosity moldable condition to a high-viscosity state with a decrease in temperature through its glass transition range (Chapter 1), bite registration materials are necessarily thermo-plastic polymers whose glass transitions occur slightly above mouth temperature. Rosin, resins, and waxes are thermoplastic polymers that, when combined with appropriate filler particles, can be formulated into convenient bite registration materials. Simple heating of these materials only to a temperature that makes them sufficiently fluid to capture the occlusal registrations of teeth in opposing arches, followed by cooling to mouth temperature, are required for their use. Clinical use of an excess amount may cause the impression material to flow into an undercut, with subsequent unwanted plastic deformation or fracture upon removal from the mouth.
Alginate Hydrocolloid
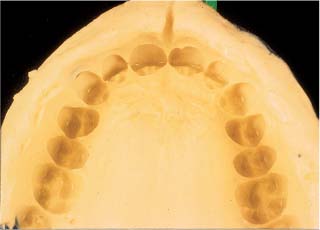
Water, potassium alginate, and calcium sulfate dihydrate react to form a calcium alginate gel that is insoluble in water, and thereby produce a viscoelastic material that has found extensive use as the principal component of an impression material used to obtain orthodontic study models. A substantial amount of filler, with some disinfectant, setting-time modifiers, and chemicals that counteract the inhibiting effect of the gel on the setting of gypsum, are used to provide acceptable clinical properties of this irreversible hydrocolloid impression material. All ingredients except the water are in powder form, and are mixed by the manufacturer with a glycol to prevent the small powder particles from becoming harmful dust that might be inhaled as the powder is dispensed. A list of the powder components and their purposes is provided in Table 12.1. Mixing of this powder with adequate water to provide the appropriate initial viscosity starts the setting chemical reaction, forming a gel that loosely contains the excess water. The sequence of reactions associated with the formation of the alginate gel is provided in Table 12.2. The properties of this alginate impression material are dependent on the distribution of the sizes of the powder particles in the mix, thus requiring a thorough mixing of the powder prior to dispensing from a large container. The properties of the set material are also highly dependent on the powder/water ratio used to prepare the mix. Precise measurement of the powder and water components is required to assure proper handling characteristics, little dimensional change during setting, and optimal resistance to plastic deformation upon removal from the patient’s mouth. After setting, water is easily exchanged into or out of the gel, with resultant changes in dimensional accuracy, so immediate pouring of the model is indicated. Figure 12.1 shows an example of an alginate impression.
| Components | Function |
| Diatomaceous earth or other filler | Control consistency before setting, and flexibility after setting |
| Potassium alginate and calcium sulfate | Form alginate gel |
| Potassium sulfate or other gypsum-modifiers | Counteract the inhibiting effect that the set impression has on the setting of gypsum |
| Sodium phosphate | Initially prevents the calcium ions from reacting, thus extending working time |
| Ammonium salts and chlorhexidine | Provide disinfection |
| Glycols | Render the powder dustless |
|
Other |
Provide taste and color |
From Craig, 1997
Fig. 12.1 An impression obtained with an alginate hydrocolloid impression material
Rubber Impression Materials
Synthetic oligomers of various molecular compositions and sizes are supplied for use as dental impression materials. Oligomers are long-chain organic molecules that have reactive groups capable of further molecular binding. These oligomers are viscous fluids that can polymerize further through the processes of chain lengthening and cross-linking to form a viscoelastic solid rubber. Polymerization reactions are exothermic, and dimensional contraction of the impression material occurs as a direct result of the increase in density during setting and from cooling after it is removed from the patient’s mouth.
The molecular composition of the oligomer determines its basic chemical and physical properties; however, these properties are susceptible to effects of the molecular size of the oligomer. When comparing oligomers of identical molecular composition, the smaller the size of the oligomer, the lower is its viscosity before setting and the greater the dimensional contraction during polymerization. Thus, the light-bodied impression materials, with their smaller oligomer size, register surface detail more readily and tend to displace soft tissue less than their medium-bodied or heavy-bodied counterparts. The light-bodied impression materials may also contain a smaller amount of filler particles. However, the light-bodied materials contract to a greater degree on polymerization and should be used sparingly, only where needed for improved registration of undisturbed surface detail. A compatible heavy-bodied impression material then completes the bulk of this dual-material impression and results in less overall dimensional contraction upon setting. When improved surface detail is not required, a single medium-bodied material can be used to obtain the entire impression, although greater dimensional contraction on setting can result.
Polysulfide Rubber
Mercaptan oligomer polymerizes by a condensation or step-growth process to form a visco-elastic polysulfide rubber. The hydrophilicity of mercaptan allows this material to wet the surfaces of moist oral structures and reduces the possibility of formation of air bubbles at the impression surface. Lead dioxide is the component that is usually incorporated to react with the hydrogen sulfide groups of the mercaptan oligomer and facilitate the condensation polymerization, and this ingredient can leave a dark stain on clothing and other materials with which it comes in contact. The dimensional contraction that occurs during the polymerization of polysulfide rubber requires using this material at minimal thickness, and therefore a custom-made tray is employed. The cross-linking chains that form in the polysulfide rubber are long relative to the lengths of these chains in other synthetic rubber impression materials and are not as effective in preventing viscous flow upon removal of the material from the patient’s mo/>
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses