Chapter 13
Intracanal Medication in Root Canal Disinfection
Lea Assed Bezerra da Silva, Raquel Assed Bezerra da Silva and Paulo Nelson-Filho
Department of Pediatric Dentistry, School of Dentistry of Ribeirão Preto. University of Sao Paulo, Riberao Preto, Brazil
Nestor Cohenca
Department of Endodontics and Pediatric Dentistry, University of Washington, Seattle, WA, USA
Introduction
Considering that apical periodontitis is a disease caused primarily by microorganisms and/or their by-products infecting the root canal system (1), the key for the success of endodontic treatment and apical repair is infection control (2–4). It is known that microorganisms aggregated in well-established biofilms are more resistant to killing (5) and that residual endodontic infection can cause the persistence of apical periodontitis (4). In a clinical study, Sjogren et al. verified that postendodontic treatment success rate was significantly lower in teeth with apical periodontitis compared with teeth without the disease (6). Therefore, the primary goal of the endodontic treatment in teeth with chronic apical periodontitis and a long-term infectious process should be eliminating the microbial infection from the root canal system (2, 7–10). In addition, root canal treatment should be done as early as possible because apical periodontitis causes tissue destruction, involving loss of periodontal ligament fiber attachment, concomitantly with cementum and bone resorption (Figure 13.1a,b), which might culminate in tooth loss (11).
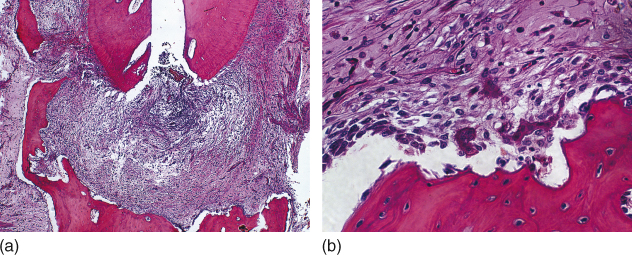
Figure 13.1 Apical periodontitis: tissue destruction, loss of periodontal ligament fiber attachment, cementum, and bone resorption (a, b). Hematoxilin and eosin.
It has been discussed whether the biomechanical preparation alone can eradicate endodontic infection in teeth with apical periodontitis. In these cases, microorganisms are disseminated throughout the root canal system, namely main canal, lateral, accessory and secondary canals, dentinal tubules (Figure 13.2), cemental lacunae (Figure 13.3), apical delta ramifications (Figure 13.4a,b), apical foramen, apical erosions,

Figure 13.2 Microorganisms are disseminated throughout the dentinal tubules in teeth with apical periodontitis. Brown and Brenn staining.
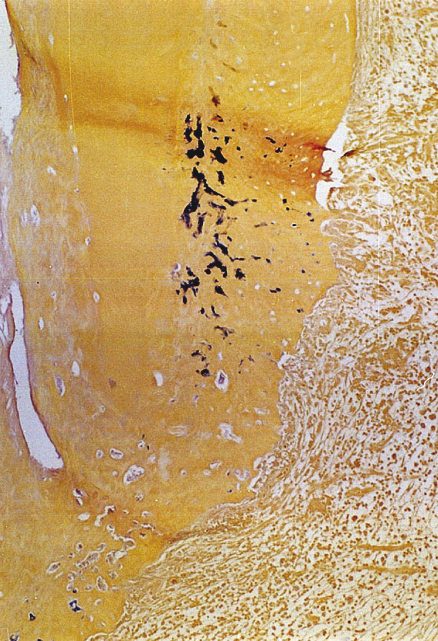
Figure 13.3 Microorganisms found in cemental lacunae in teeth with apical periodontitis. Brown and Brenn staining.
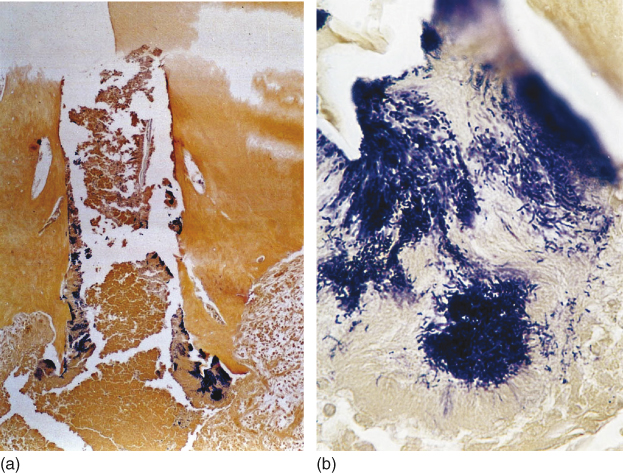
Figure 13.4 (a, b) Microorganisms found in apical delta ramifications in teeth with apical periodontitis. Brown and Brenn staining.
and are even organized as biofilms on the external surfaces of the apical root third (Figure 13.5a,b)(12, 13), which are areas inaccessible to the biomechanical preparation and to the body defense system (14–18).
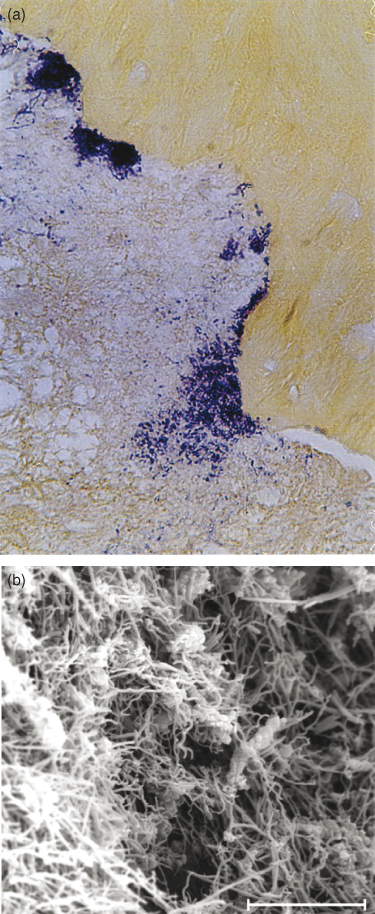
Figure 13.5 Microorganisms organized as biofilms on the external surfaces of the apical root third. Brown and Brenn staining (a) and scanning electronic microscopy (b).
Historically, different substances and medications have been used as intracanal medication between endodontic treatment sessions to assist in the control of microbial infection, including calcium hydroxide-based pastes, polyantibiotic pastes, formalin tricresol, camphorated paramonochlorophenol, and chlorhexidine alone or in combination with other substances.
At the same time, studies using microbial culture and biomolecular techniques in permanent teeth have established that the infection in teeth with pulp necrosis and apical periodontitis is of polymicrobial nature, with predominance of anaerobic microorganisms (19–21), mainly Gram-positive. bacteria (20). These characteristics have also been demonstrated in primary teeth (22, 23).
In addition to generating toxic products and by-products to the tissues, Gram-positive bacteria present as a major component of its cell wall endotoxin, also known as LPS following its lipopolysaccharide nature (24, 25), that acts as one of the most important virulence factors of these microorganisms (26–29). Bacterial endotoxin is released after the death or multiplication of microorganisms and causes a series of biological events that activate immunocompetent cells. In sequence, this activation leads to the release of several proinflammatory cytokines and mediators that are responsible for establishing an inflammatory response (24, 30–33), osteoclastogenesis and cemental and bone resorption (34–36), thus contributing to the genesis (Figure 13.6), development, and persistence of apical periodontitis (25, 37). A positive association has also been observed between the amount of bacterial endotoxin in the root canals and the presence of clinical symptoms, such as pain, tenderness to percussion and palpation, edema, and purulent exudate (38).
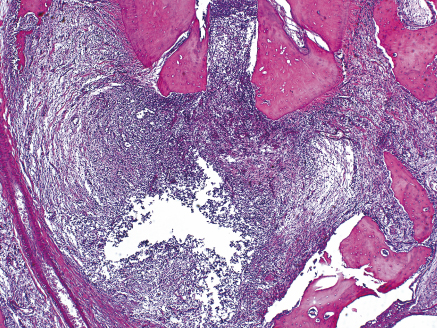
Figure 13.6 Apical inflammatory response and osteoclastogenesis, resulting in apical periodontitis development. Hematoxilin and eosin.
Bacterial endotoxin has long been recognized as a potential stimulator of several host’s cells via receptors, such as lipopolysaccharide-binding protein (LBP), CD14 receptor (39), and toll-like receptors (TLRs) (39, 40), causing the expression of proinflammatory cytokines and amplification of the host’s response. The bacterial endotoxin acts on macrophages and other cell types (24) by stimulating the expression of the tumor necrosis factor (TNF) (41), interleukin-1, interleukin-6 (30), and interleukin-8 (30, 42). In addition, bacterial endotoxin acts as a potential inductor of nitric oxide production (13).
Bacterial endotoxin also activates the Hageman factor (coagulation factor XII) (43), arachidonic acid metabolism, and complement system (44, 45), and acts as a pyrogen agent inducing fever (46), being involved in inflammatory response events, such as the increase of vascular permeability, neutrophil and macrophage chemotaxis, and release of lysozymes and lymphokines (44). It has been suggested that the LPS-mediated sensitization of nociceptors is one of the mechanisms responsible for the pain associated with endodontic and apical infections (47).
In addition to causing inflammatory reaction, bacterial endotoxin adheres to mineralized tissues, stimulating bone resorption (37), synthesis, and release of osteoclast-activating cytokines (36, 48), and osteoclastogenesis (36). Thus, in teeth with apical periodontitis, the use of an intracanal medication between sessions is indicated to assist in the elimination of endodontic infection and inactivate bacterial endotoxin. Several materials with antimicrobial activity have been evaluated to inactivate the bacterial endotoxin, other bacterial cell wall components with and without effective results, including calcium hydroxide (33, 36, 37, 49, 50), polymyxin B (51), formocresol (52), chlorhexidine (53) Er:YAG laser (54), ultrasound (55), ozonized water (56), and sodium hypochlorite at different concentrations (53, 57, 58).
The chances of having bacterial endotoxin and viable microorganisms in the canal at the filling stage are greater in single-session endodontic treatments. Theoretically, most of the residual bacteria would be killed because of the contact of the filling material or would remain entombed inside the dentinal tubules and die from starvation following scarcity of nutrients. Also, considering the complex canal anatomy and the small number and low pathogenicity of the residual bacteria, it is assumed that those who survived would not reach the apical tissues. However, it should be considered that fillings do not provide hermetic apical and cervical seals. In addition, residual microorganisms could reach the periapex through the interface between the filling material and the canal walls, and through the dentinal tubules, apical cementum, and apical delta canals. Microorganisms may also persist after single-session endodontic treatment in aggregates adhered to cementum surface (apical biofilm). For these reasons, a significant correlation has been demonstrated between endodontic failures and microorganisms surviving after root canal filling.
Traditionally, the root canal treatment of teeth with necrotic pulp and apical periodontitis has been done in multiple sessions with the use of an interappointment intracanal medication being accepted as a safe and effective therapy (59). The philosophy of completing the root canal treatment of these teeth in one visit has been proposed only to shorten treatment duration and is based on achieving similar outcome rates in clinical and radiographic evaluations after single-session treatments (60–65). However, this is still a controversial topic within the endodontic literature (65) mainly considering the relatively short follow-up periods of some studies (63, 64). In addition, some of those who adopt single-session endodontic treatments consider partial apical repair in asymptomatic teeth as success, which increases their treatment success rates.
It is incontestable that the persistence of apical periodontitis or even partial posttreatment radiographic repair indicates the existence of residual infection in the root canal system or on the external apical root surfaces. This condition may impede the occurrence of complete repair, resulting in the maintenance of a chronic apical inflammatory infiltrate and formation of areas of cemental and bone resorption (Figure 13.7).
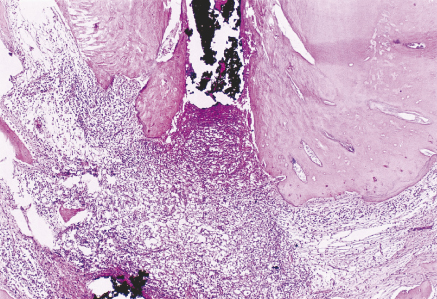
Figure 13.7 Persistence of apical periodontitis following root canal treatment indicating the existence of residual infection in the root canal system or on the external apical root surfaces. Hematoxilin and eosin.
It is known that clinical silence may remain for several months or years. This condition together with radiographic apical repair, often partial, refers to an evolutive phase of the healing process without recovery of the lamina dura, and therefore it does not represent an ideal histological repair. In a histopathological analysis, apical lesions with partial radiographic resolution present accentuated tissue disorganization and persistence of inflammatory response events.
Following the discussion above, we can conclude that the intracanal medication of choice should be an antiseptic medication without adverse effects to the apical tissues and the capacity to stimulate the occurrence of repair.
Intracanal medication—definition
Intracanal medication can be defined as a medication or substance used after biomechanical preparation of root canals between treatment sessions to
- eliminate the endodontic infection and the microbial proliferation in the root canal system and neutralize the bacterial endotoxin in teeth with pulp necrosis and apical periodontitis;
- reduce the intensity of apical and apical inflammatory responses;
- act as a physical and chemical barrier, preventing canal reinfection and minimizing coronal leakage through the temporary restoration; and
- increase the pH of the region.
Basic requisites for an interappointment intracanal medication
A substance or medication to be used as an interappointment intracanal medication should (66)
- have a broad spectrum of antimicrobial activity;
- eliminate residual microorganisms in the canals after instrumentation and neutralize bacterial endotoxin;
- present physicochemical properties that permit its diffusion through the root canal system and sufficient action time to reach the microorganisms at different canal sites;
- reduce apical tissue inflammation;
- act as a barrier against coronal leakage through the temporary restorative material;
- assist in the control of persistent exudation; and
- have tissue compatibility to favor the repair without causing additional irritation to the apical tissues.
Materials used as interappointment intracanal medication
Calcium hydroxide
Contemporary endodontics seeks substances that combine antibacterial and anti-inflammatory properties and capacity to induce mineralized tissue formation in such a way that the interrelation of these properties results in a medication with a beneficial effect to the repair of the apical tissues.
Calcium hydroxide is an odorless white powder with molecular weight of 74.08 and low solubility in water (∼1.2 g/l at 25 °C, decreasing with the increase of temperature) (67). Its coefficient of dissociation of 0.17 permits a slow and controlled release of calcium and hydroxyl ions (68). The low solubility is a favorable characteristic because a long period is necessary before the solubilization of the material in direct contact with the tissue fluids. Calcium hydroxide has an alkaline pH (∼12.5–12.8) and is chemically classified as a strong base (69). Its main properties—antimicrobial activity and capacity of inducing mineralized tissue deposition (70)— is a result of its ionic dissociation.
Calcium hydroxide has been traditionally considered as the intracanal medication of choice (2, 68, 71) because of its simultaneous multiplicity of actions that include antimicrobial activity (14, 72–75), even against Enterococcus faecalis (76, 77); indirect antimicrobial action by environmental carbon dioxide absorption (78) and consequent stimulation of apical and apical repair; assistance in the control of apical exudation (74, 79) following its hygroscopic propriety; capacity to dissolve necrotic material (80); reduction of the amount of matrix metalloproteinases (81); release of inflammatory mediators (74) and the inactivation of the lipoteichoic acid from E. faecalis (50); anti-inflammatory activity (82); participation in mineralized tissue formation (16, 83, 84), with alkaline phosphatase activation and collagen synthesis (85); increase of extracellular calcium levels, inducing differentiation of periodontal ligament cementoblasts and cementogenesis (86); and, most importantly, tissue compatibility (16, 87–89) (Figure 13.8a–c).
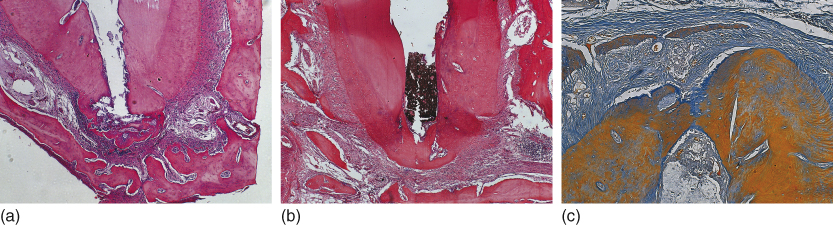
Figure 13.8 Mineralized tissue formation (cementogenesis) as a result of calcium hydroxide root canal dressing and filling (a, b, c). This response indicates tissue compatibility. Hematoxilin and eosin and Mallory Trichrome.
Calcium hydroxide also has the capacity to penetrate into the dentinal tubules (90), increasing the pH on the root periphery. This property contributes to the regression of resorption processes because the alkalinization of regions with acidic pH creates unfavorable conditions to the action of osteoclasts. In addition, calcium hydroxide has been widely used in dentistry to stimulate mineralization because of its dissociation into hydroxyl and calcium ions. The hydroxyl ions are responsible for maintaining an alkaline pH while the calcium ions have a direct effect on extracellular matrix mineralization (91). Furthermore, the increase of intracellular calcium levels activates the calcium/calmodulin-dependent pathways, which regulates osteoblast differentiation via phosphorylated extracellular signal-regulated kinases (ERK-1/ERK-2) and c-fos protein expression (92).
An important aspect to be considered is that, to date, calcium hydroxide is the only intracanal medication that presents effective results on bacterial endotoxin inactivation (35, 36, 41, 49, 58, 93–95), without causing damage to tissue repair (33).
On the basis of all these considerations and in agreement with health promotion principles, calcium hydroxide should be the medication of choice when the use of an interappointment intracanal medication is required.
Vehicle
Calcium hydroxide does not have good physical properties presenting low flow, viscosity, and adherence to canal walls, in addition to being radiolucent. Therefore, to be used in the form of paste under clinical conditions, calcium hydroxide must be combined with an aqueous, viscous, or oily vehicle, for example, distilled water, saline, anesthetic solution, methylcellulose, cresatin, polyethylene glycol (PEG), propylene glycol, and olive oil. On the other hand, the potential interferences of the different vehicles on the action of calcium hydroxide must be carefully examined because the type of vehicle determines the velocity of ionic dissociation and the capacity of paste solubilization (96), which might alter its antimicrobial activity (97), tissue compatibility (87), and the mineralized tissue-inductive capacity (98).
Aqueous vehicles permit a rapid ionic dissociation. Nevertheless, viscous vehicles like PEG 400 or propyleneglycol allow for a slow but sustained release of hydroxyl ions, maintaining an alkaline environment for a longer period (98). At the same time, it has been suggested that such pH elevation resulting from the release of hydroxyl ions could start or favor the mineralization process (90).
The use of a commercial calcium hydroxide paste in aqueous vehicle supplemented with blood salts, Calasept® (Speiko, Darmstadt, Germany), in the endodontic treatment of immature teeth with vital pulp resulted in the formation of mineralized tissue barriers at the apical level and the absence of inflammatory and normal periodontal ligament thickness (98).
A commercial calcium hydroxide paste using PEG 400 as vehicle (Calen®; S. S. White; Artigos Dentários Ltda., Rio de Janeiro, RJ, Brazil) has been shown to maintain calcium hydroxide for a longer time in the region of interest, prolonging its action, decreasing its solubilization in the fluids, and increasing its capacity of penetrating the tubular root dentin (98). Calen presents pH around 12.4 (87), high antimicrobial activity and tissue compatibility (16, 87). Theoretically, the low PEG 400 dispersion results in slower dissolution rates of the paste with consequent lower release of calcium ions compared with the use of distilled water as a vehicle. This is relevant, considering the changes of intracanal medication during the treatment and the need of maintaining an alkaline pH and calcium and hydroxyl ion release.
The mild tissue aggression caused by Calen could be explained by the fact that the hydroxyl ions released from this paste do not remain free for a long time because they react with the hydrogen ions released from PEG 400 ionization in water (neutralization reaction). In the commercial calcium hydroxide paste with distilled water (Calasept), on the other hand, the hydroxyl ions released from calcium hydroxide dissociation are not neutralized because there are no free hydrogen ions in the solution. However, even though Calasept causes an initially more severe tissue aggression than Calen, both pastes have been shown to offer a similar tissue repair in the later periods of evaluation (87, 98).
Aqueous and viscous vehicles should be preferred to calcium hydroxide-based intracanal medications. Oily vehicles are not indicated because they impede ion dissociation from the paste (99).
Endodontic treatment of teeth with apical periodontitis
Single session × multiple sessions
While some clinical and radiographic studies have found no difference between the single- and two-session endodontic treatments using a calcium hydroxide-based intracanal medication (60, 62, 63, 65, 100–108), or that the single-session treatment can be more effective (64), other clinical, radiographic and histopathological studies have found higher success when an intracanal medication was placed between sessions (16, 86, 109–117). A fact that is worth mentioning is that in most studies that found no difference between single- and two-session treatments, the intracanal medication was left in the canal for only 7 days, a period that is considered insufficient (118), as is discussed later in this chapter.
As emphasized before, another relevant aspect to be discussed is that after completion of an endodontic treatment, we should be aware that the absence of postoperative pain or “clinical silence” per se does not necessarily indicate treatment success. For both primary and permanent teeth with pulp necrosis and chronic apical periodontitis, no endodontic treatment can be considered as successful based only on the findings of clinical and radiographic examinations. The results of tomographic (clinical trials) and histopathological (research studies) analyses must be computed as well, as several authors have found higher success rates of root canal treatment performed with the use of a calcium hydroxide-based intracanal medication between sessions compared with one-visit treatment (16, 86, 112, 115, 117). Endodontic techniques that reach success at all these levels should be the ones of choice for use in clinical practice.
Intracanal medication: how to perform?
In order to obtain a deeper penetration of the paste into the dentinal tubules, the instrumented root canals should be filled with EDTA for 3 min under agitation with a K-file to remove the smear layer (119). Next, the canals should be copiously irrigated, dried with paper points, and filled with a calcium hydroxide-based paste in aqueous or viscous vehicle with slight apical overflow to reach the apical biofilm.
Duration of calcium hydroxide therapy
A minimal period of 14-21 days is necessary for calcium hydroxide to diffuse through the regions that may harbor microorganisms responsible for endodontic failure (e.g., dentinal tubules, apical cementum, and accessory/collateral canals) (112), increasing the pH of the external apical root surface and killing microorganisms and eliminating bacterial endotoxin from these regions (112, 118, 120, 121).
Although the usual duration of an interappointment intracanal medication is 7 days, this time is not sufficient to eliminate residual microorganisms from the root canal system. The interappointment intracanal medication should be maintained for, at least, 14 days (112), which is the time necessary for a greater release of calcium and hydroxyl ions (118) and for these ions to penetrate deeply into the entire dentin thickness, reaching the external apical root cementum.
From a histopathological standpoint, the best results of apical and apical repair are obtained after 15 and 30 days of calcium hydroxide therapy, while the worst results are observed when an intracanal medication is not used or is left for only 7 days. For this reason, the calcium hydroxide paste should be used as an interappointment intracanal medication in teeth with pulp necrosis and apical periodontitis for no less than 14 days.
From a mechanical and clinical perspective, new evidence has demonstrated that removal of calcium hydroxide by additional instrumentation in a second appointment may lead to canal transportation with higher incidence in curved canals (122), and residual medication might remain in the root canal system (123). Furthermore, calcium hydroxide has recently been associated with reduced fracture resistance of the dental hard tissues when applied for more than 30 days (124, 125). The intracanal placement of calcium hydroxide weakened the microtensile fracture strength of teeth with an average of 0.157 MPa per day (126). The authors also reported a statistically significant difference between 7 days (45.7 MPa) and 28 days (35.6 MPa) and also between 7 and 84 days (31.8 MPa).
Periapical radiograph × computed tomography
Apical radiolucency is the main suggestive sign of apical periodontitis. Conventional and digital Apical radiographs are the main auxiliary resources for diagnosing and following up apical periodontitis resolution after root canal treatment. It is generally accepted that posttreatment follow-up of apical periodontitis for confirmation of complete healing requires at least 4 years of radiographic examination (127–129). Nevertheless, radiographic findings are frequently divergent from clinical or microscopic features in teeth with apical periodontitis. These outcomes indicate that, in many situations, apical periodontitis may persist for several years after root canal filling, even in the absence of clinical and radiographic signs and symptoms (111).
These divergences result from the fact that the radiographs produce two-dimensional images of a three-dimensional structure, which impedes the detection of apical lesions confined to the cancellous bone, especially in areas with thicker cortical bone (130–132). In addition, apical radiographs provide images of teeth in the sagittal plane (mesiodistal direction), and images in the coronal plane cannot be obtained. This fact is a limitation for evaluating the buccolingual extension of the apical lesion and determining cortical bone tissue resorption level (133–135).
Computed tomography (CT) has been widely used in medicine since 1970 and it was gradually introduced to dentistry, more specifically to endodontics, in 1989 (136). However, the use of this computed-assisted imaging technology has become widespread in endodontics only in the past few years thanks to the development of a high-resolution tomography called cone beam computed tomography (CBCT) (137–141). The importance of CT has been demonstrated in previous studies in which apical radiolucencies identified by CT were not detected by apical radiography (142–144). Computed tomography may also produce data about pre- and postendodontic treatment bone mineral density (145).
In a previous study, comparison of the size of experimentally induced apical periodontitis obtained with apical radiography and CBCT scans showed that the tomographic evaluation detected lesions with larger mesiodistal extension while radiographic evaluation underestimated the size of lesions (146) (Figure 13.9a,b). In addition, the sensitivity and accuracy of CBCT for detection of apical periodontitis has been shown to be superior to that of conventional apical radiography (147, 148), considering microscopic evaluation as the gold standard (149). The divergence between CT and conventional apical radiography could be because of the superimposition of three-dimensional structures in the two-dimensional radiographic image, which can mask the real size of the lesion. Conversely, CBCT can provide a sequence of serial slices that allow for determining the real dimension of apical periodontitis, using a thin slice containing only the cancellous bone region (135, 139, 142, 150, 151).
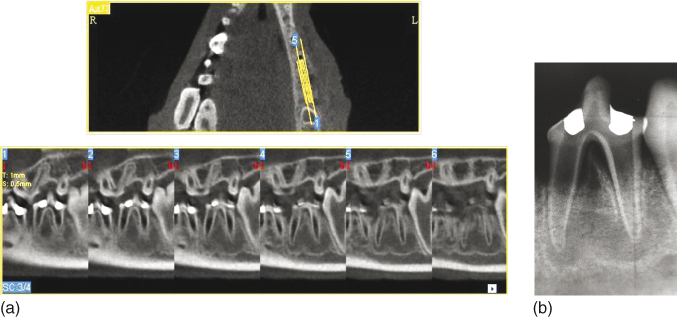
Figure 13.9 Large apical periodontitis detected by cone beam computed tomograph (CBCT) evaluation (a). Apical radiograph of the same tooth showing the underestimated mesiodistal extension of the lesions (b).
The superiority of CBCT in the detection and evaluation of apical periodontitis size has been demonstrated when apical lesions with mean mesiodistal diameter of 2.8 mm, not detected by conventional apical radiography, could be detected by tomographic evaluation (142). Another study showed that during the development of apical periodontitis, the radiographic examination did not detect radiolucent areas 14 days after root canal contamination, while CT detected apical radiolucency in 33% of the specimens. These results were similar to those obtained 21 days after contamination, when conventional apical radiography and CT detected apical lesions in 47% and 83% of the specimens, respectively (143). In teeth subjected to endodontic retreatment, superiority of CT over conventional apical radiography for detection of apical lesions has been attributed to the lower possibility of false-negative results (152).
According to Paula-Silva et al. (149), teeth with apical disease subjected to single-visit endodontic treatment evaluated by CBCT, apical radiography, and histological analysis (gold standard) showed discontinuity of the lamina dura and radiolucent areas suggestive of apical periodontitis. The fact that the lesions in these teeth were larger compared with those of the other groups evaluated in the study allowed all diagnostic methods to have a similar performance in lesion detection, which also occurred in the teeth with experimentally induced apical periodontitis not subjected to root canal treatment. On the other hand, in the teeth with apical periodontitis subjected to endodontic treatment using a calcium hydroxide intracanal medication, discontinuity of the lamina dura and presence of radiolucent areas suggestive of apical periodontitis were detected in 71.4% of the specimens by apical radiography, while CBCT allowed this detection in 100% of specimens, similar to the microscopic evaluation. These divergent tomographic and radiographic results could be because of the fact that the lesions in this group had a smaller mesiodistal extension with larger amount of surrounding bone tissue increasing the superimposition of sound bone structure (Figure 13.10a,b).
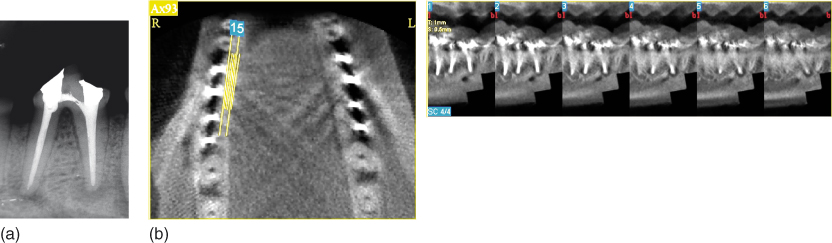
Figure 13.10 Persistent apical periodontitis detection following root canal treatment is divergent in cone beam computed tomograph (CBCT) and radiographic evaluations. Superimposition of sound bone structure indicated smaller mesiodistal extension in the radiograph (a) compared with the CBCT scan (b).
A previous study showed a positive correlation between microscopic and microtomographic measurements of apical lesion size, as well as between the area and volume of the lesions measured by microtomography (135). On the other hand, conventional apical radiography has shown low reliability to detect the presence of apical lesions and the reduction of lesion size, which could be attributed to the fact that the buccolingual expansion of the lesion in the cancellous bone cannot be visualized in a sagittal plane (153–157). In these situations, the increase in the lesion size can be revealed only by volumetric measurements, as provided by CT (130, 158).
In experimental studies, unfavorable outcomes have been found more frequently in teeth subjected to a single-session endodontic treatment compared with those in which a calcium hydroxide intracanal medication was used between two or more treatment sessions (16, 111, 112, 115, 117). On the other hand, clinical and radiographic studies in humans for the posttreatment follow-up of root canals have found no difference in the outcome of endodontic treatment completed in one or two-session procedures (60, 101, 102, 110, 159, 160), possibly because of the limitations of clinical research design, especially regarding the sample size and the methods used for apical lesion detection.
There are divergences in the literature over the time required to confirm radiographic success or failure after endodontic treatment. Currently, the European Society of Endodontics recommends a clinical-radiographic follow-up of 1 year after root canal filling, with annual evaluation during a period of 4 years in order to determine treatment success or failure (161). The American Association of Endodontists recommends a minimum of 4–5 years for root canal treatment follow-up (162). Considering that apical lesions confined to the cancellous bone might pass undetected radiographically during or after the follow-up period (130–132, 153–156, 163), the use of CT might establish new follow-up periods to determine the tomographic success of root canal treatment. The fact that CT is useful for determining the actual lesion size and its spatial relationship with the different anatomic sites indicates that this imaging method should be considered a valid option for the follow-up of apical lesion repair after root canal treatment (164). Other advantages of CT over conventional apical radiography include measurement of bone tissue thickness at different planes, precise location of the mandibular canal and its relation to the tooth apexes, differentiation of lingual from buccal root canals by coronal tooth slicing, and detection of vertical root fractures in endodontically and nonendodontically treated teeth (135, 139, 142, 150, 165–169).
Combination of calcium hydroxide with chlorhexidine
Chlorhexidine is a cationic bisbiguanide. Structurally, its molecule consists of two symmetrical chlorophenol rings (4-chlorophenyl) and two bisbiguanide groups united by a central hexamethylene chain that provides its hydrophilic and hydrophobic properties (170). It presents a broad spectrum of action (171) against gram-positive, gram-negative bacteria, aerobes, anaerobes, yeasts, fungi, and viruses (10, 172–176), and binds to the mineralized tissues, with prolonged release at specific therapeutic levels (71, 177, 178). The antimicrobial activity of chlorhexidine is the result of its ionic interaction with the negatively charged bacterial cell surfaces, causing cell wall damage, enzymatic inhibition, and irreversible loss of cytoplasmic content (176). An important property of chlorhexidine is its substantivity (179–182), which permits a sustained residual effect without causing microbial resistance (177, 182).
The use of calcium hydroxide in combination with chlorhexidine has been investigated as an intracanal medication (17, 23, 77, 82, 83, 115, 182–186). Although chlorhexidine alone is not capable to inactivate the bacterial endotoxin (53, 58, 95, 187) and dissolve necrotic tissues (188), such a combination could produce a synergic antimicrobial effect (7, 77, 171, 183) and increase the antimicrobial action of the medication against resistant microorganisms (7, 71, 172, 189–192). Even at low concentrations, the efficacy of chlorhexidine against the most prevalent microorganisms in endodontic infections has been demonstrated (193). The potential additional benefits of this combination for the root canal treatment of teeth with chronic apical periodontitis has raised scientific interest and has been investigated in-vitro, ex-vivo, and in-vivo (23, 115, 171, 183, 190, 194).
On the other hand, it has been demonstrated that the addition of chlorhexidine does not increase the antimicrobial capacity of calcium hydroxide pastes (195), although the antimicrobial activity of calcium hydroxide is not affected. Ercan et al. compared the in-vitro antimicrobial activity of calcium hydroxide p.a. in combination as well as alone with 2% chlorhexidine against E. faecalis and concluded that chlorhexidine alone was significantly more effective than when combined with calcium hydroxide (184).
In this regard, a reasonable question to be posed could be why the antimicrobial activity of calcium hydroxide is maintained and the antimicrobial activity of chlorhexidine is reduced when these materials are combined in a single medication. A possible answer could be related to the fact that the antimicrobial properties of calcium hydroxide are directly related to its pH (196, 197), that is, its capacity to release hydroxyl ions. According to Haenni et al., the pH of the paste is not altered with the addition of chlorhexidine because calcium hydroxide is a highly alkaline substance (195). The combination of materials does not cause alterations on ion release, maintaining the alkaline pH and, consequently, calcium hydroxide’s antimicrobial activity. On the other hand, the combination with calcium hydroxide (pH ∼12.5) inhibits the antimicrobial activity of chlorhexidine probably because this bisbiguanide is deprotonated at pH 10 or higher, which results in the reduction of solubility and alterations on the interaction with the microbial surfaces because of changes on molecule charge (198). According to Gomes et al., the antimicrobial action of chlorhexidine is reduced when it is combined with calcium hydroxide possibly because of chlorhexidine precipitation at high pH conditions (199). Further physicochemical and antimicrobial studies are required to elucidate the mechanism involved in the potential loss of chlorhexidine efficacy when combined with calcium hydroxide.
An important and conflicting aspect refers to the toxicity of chlorhexidine used alone as an intracanal medication. Although some authors have claimed that chlorhexidine presents low toxicity (170, 200) and beneficial effects (201–203), not affecting collagen synthesis or surgical wound repair, other authors have reported that chlorhexidine presents toxic effects to various cell types, according to its concentration (82, 83, 204–206). Delay of granulation tissue formation (207), induction of microcirculatory alterations (208), and delay of tissue repair (209–211) have also been associated with the use of chlorhexidine. Some studies have demonstrated that subcutaneous injection of chlorhexidine at different concentrations induced inflammatory tissue reactions (200, 212).
There have also been reports that the use of chlorhexidine intracanal medication could affect protein synthesis (213, 214) and cell proliferation (215) at different levels; cause endoplasmic reticulum (ER) stress as a consequence of protein accumulation in the ER cisterns; induce apoptotic or necrotic cell death via ER stress; and increase cellular stress-related protein expression (216).
It should be emphasized that these effects are dose-dependent (211). In a previous study, the addition of 0.4% chlorhexidine to a commercial calcium hydroxide paste (Calen paste) did not result in immunostimulatory effects, with no increase of nitric oxide (NO) production and consequently no cytotoxicity (82). Nevertheless, in another study, the same calcium hydroxide paste added with 0.4% chlorhexidine did not increase the osteogenic potential (83).
Regarding tissue compatibility, the use of a calcium hydroxide and 1% chlorhexidine paste as intracanal medication resulted in a significant reduction in the mean size of the radiographic image and improvement in the histopathological healing of apical radiolucent images in comparison with single-session endodontic treatment of dog’s teeth. However, although the use of this paste provided remarkably better results compared with the completion of root canal treatment in a single session, some adverse effects associated with the chlorhexidine concentration (1%) in the paste were observed (115).
The results of studies obtained so far indicate that the incorporation of chlorhexidine to calcium hydroxide-based intracanal medications does not offer any additional beneficial effects and is therefore unnecessary.
Triple antibiotic paste
Antibiotics have been used as intracanal medication in root canal treatment since the 1950s (217). However, local application of antibiotics in endodontics has been restricted because of the polymicrobial nature of apical alterations and the risks of adverse effects, such as hypersensitivity, toxicity, development of microbial resistance, and favorable conditions for fungal growth (218–220). In spite of this, the interest for using a combination of antibiotics as an intracanal medication has reemerged with the introduction of a paste containing three antibiotics. It has appeared as an alternative conservative treatment option, especially in young permanent teeth with immature roots, for root canal disinfection (221, 222), and revascularization of necrotic pulps (223–227).
The triple antibiotic regimen was first tested in-vitro by Sato et al. (228) followed by Hoshino et al. (229). The so-called triple antibiotic paste is prepared by mixing 20 mg of ciprofloxacin, minocycline, and metronidazole antibiotics with sterile distilled water and is used as an intracanal medication usually for 2 weeks (221).
Despite the recognized antimicrobial activity of this paste (3, 230), it should be emphasized that the use of antibiotic-based intracanal medications may result in clinical and biological side effects, including crown darkening (225, 231, 232) (Figure 13.11), development of microbial resistance (233) and allergic reactions (234–236). In addition, minocycline, one of the active components of the triple antibiotic paste, has been associated with angiogenesis inhibition (237, 238).
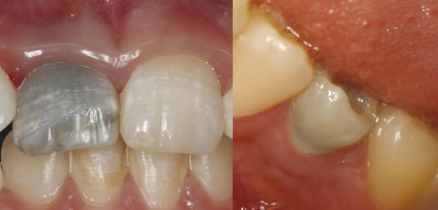
Figure 13.11 Crown discoloration as the result of a revascularization procedure performed with triantibiotic intracanal medication.
It should also be considered that the use of the triple antibiotic paste is based fundamentally on the reports of clinical cases in immature teeth with pulp necrosis (224, 226, 227) and mature teeth with pulp necrosis and extensive apical periodontitis in which conventional intracanal medication had failed (239–241).
As emphasized by Mohammadi (220) in a literature review article, indication of any medication should be preceded by evaluation of whether the expected benefits outweigh the risks involved (242). Despite the claimed clinical and radiographic success and possible undesirable side effects, few histopathological studies have evaluated tissue compatibility of the triple antibiotic paste (243, 244).
Yoshiba et al. (243) evaluated pulp tissue response to the combination of tricalcium phosphate and triple antibiotic paste in cavities prepared in monkey’s teeth (whether they were left exposed or not) to the oral cavity for 24 h. After 4 weeks, a well-defined inflammatory infiltrate was observed without evidence of mineralized tissue deposition in all teeth, regardless of the exposure to microbial contamination. This indicates that the paste promoted an effective disinfection of the pulp tissue, without destroying the healthy tissue. However, mineralized tissue formation was delayed by the mixture of antibiotics, compared with the use of a calcium hydroxide paste.
Silva et al. (244) evaluated in-vivo the apical and apical repair in immature dog’s teeth with experimentally induced apical periodontitis after root canal instrumentation and intracanal medication with the triple antibiotic paste compared with the use of EndoVac. The authors found a significantly more intense inflammatory cell infiltrate and a less advanced repair process when the paste was used (Figure 13.12a,b).
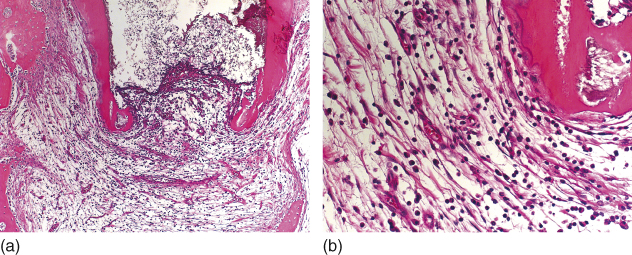
Figure 13.12 Intense inflammatory cell infiltrate in apical periodontitis of immature teeth after intracanal medication with the triple antibiotic paste (a, b). Hematoxilin and eosin.
A recent study tested the hypothesis that intracanal medications at high concentrations would be toxic to the human stem cells of the apical papilla (245). The authors concluded that the antibiotics at high concentrations present in the triple antibiotic paste had a detrimental effect on cell survival and that calcium hydroxide at all tested concentrations was conducive with the survival and proliferation of the human stem cells of the apical papilla. These results reinforce the importance of using intracanal medications at concentrations that produce a bactericidal effect without harming the dental papilla stem cells in order to permit regenerative endodontic procedures. It seems reasonable to assume that as root canal disinfection can be achieved with the use of biological medications that do not cause side effects, the use of the triple antibiotic paste is undesirable and unnecessary.
Tricresol formalin/formocresol
Tricresol formalin is a formaldehyde and cresol-based compound that, because of its chemical bond with proteins, has a bactericidal action but is also a highly irritating substance to live tissues. This medication acts both by direct contact and at distance, reaching the apical tissues and causing adverse reactions. Formaldehyde recognizably causes strong harmful effects in contact with the cells (246).
Although formaldehyde-based materials had been largely used in dentistry, the International Agency for Research on Cancer (IARC), a branch of the World Health Organization, issued an official statement in />
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


