13
Hard Tissue Reconstruction
INTRODUCTION
Successful bone continuity restoration is the basis for the success of the majority of reconstructive surgery procedures in the oral maxillofacial skeleton. There are a number of preoperative, intraoperative and postoperative considerations the surgeon must be aware of with the use of bone grafts (transfer of free cancellous, cortical, or corticocancellous bone without a blood supply), bone flaps (bone pedicled or transferred free with its own vascular system), or bone substitutes (growth factors, allogeneic, alloplastic, and xenogenic materials). Knowing the potential risks and complications with each method can provide effective informed consent and allow measures for quality control. Ultimately surgeons must provide the best possible care to the patients by minimizing known risks and complications, and having a clear understanding of their effective management.
CONTRIBUTORY PATIENT FACTORS IN COMPLICATIONS
Preoperative Comorbidity
Primary reconstruction with nonvascularized bone graft at the time of pathological resection or during trauma reconstruction, although ideal, is not always undertaken for several reasons. Occasionally the added time or blood loss from harvesting the graft may be limiting factors in certain patients. Bone graft harvesting may be associated with increased risk of surgical site blood loss perioperatively and in the postoperative period that can cause adverse patient outcomes. Risks from significant blood loss especially in older or medically compromised patients include perioperative or postoperative cardiopulmonary complications such as myocardial infarction or cardiac arrhythmias, or even neurologic sequelae, such as stroke. In addition, blood transfusions harbor the risk of several adverse reactions that may even be life threatening, while aggressive crystalloid or colloid resuscitation may add to cardiopulmonary system’s stress. Intraoperative hemostasis is essential for minimizing these risks. A complete medical history including cardiopulmonary history and functional status in the preoperative period can provide accurate risk assessment and determine if any additional evaluation is necessary.1–5 While most grafting procedures are low risk in their overall morbidity and mortality, the frail, the elderly, and the medically unfit should all be carefully evaluated and optimized preoperatively to ensure a safe treatment can be performed with minimal adverse effects. This is especially true in cases of elective reconstructive procedures that can be delayed for a period of time to maximize patient health. In cases of more urgent surgery, preoperative evaluation and discussion of risks with both the patient and anesthesiologist can allow for appropriate care and avoidance of adverse outcomes.
Risks of Blood Transfusion
With many maxillofacial surgical procedures, and especially those requiring bone reconstruction, there may be need for blood transfusion in the perioperative period. In the past, patients were transfused more liberally in an attempt to minimize major cardiopulmonary complications; however, good supportive evidence for this practice did not exist. It is well known that numerous risks exist with the transfusion of blood products including: disease transmission, systemic inflammatory response syndrome (SIRS), wound infection, sepsis, pneumonia, acute lung injury (TRALI), acute respiratory distress syndrome (ARDS), extended length of hospitalization, and increased post-transfusion complications.6–10 Some additionally have cited that there may exist an immunomodulation effect on the host that may increase the risk of cancer recurrence, though it is impossible to define the precise risk involved.11–27 Currently it is accepted that transfusions do offer some beneficial actions with improved oxygen delivery; however, this should be limited to use primarily in symptomatic patients with hemoglobin levels below 7 gm/dl. There is significant controversy on acceptable transfusion guidelines for cardiovascular and cerebrovascular compromised patients based on overall risk. While some suggest hemoglobin levels between 8 and 10 gm/dl can be tolerated by these patients, others believe transfusions at these levels are acceptable to prevent adverse outcomes.6,7 Excess blood loss and/or the need for transfusions prolong postoperative recovery with risks associated with increased hospitalization (pneumonia, hematoma, wound infection and breakdown, thromboembolism) among others.28
Meticulous hemostasis to the soft tissues from the donor site, along with use of adjunctive agents can effectively minimize procedural and postoperative blood loss. Medullary bleeding has often been minimized with the use of bone wax applied directly on the exposed cancellous marrow. There have been reports of granulomas at the site of bone wax use as well as possible delayed infections29–37 The use of other hemostatics such as oxidized cellulose (Surgicel® Ethicon, www.ethicon.com) can prove beneficial, but are also known to cause nerve paresthesias when applied directly on or in immediate vicinity to nerves.38–41 Newer resorbable wax substitutes are also available for use (Ostene®, Ceremed, www.ostene.com).
There has been interest in utilizing perioperative erythropoietin in cases where patients are anemic preoperatively or significant blood loss is anticipated to minimize the risk of blood transfusions.25,42 This is especially true for patients whose religious beliefs prevent them from receiving autologous blood transfusions.43,44 Further trials are necessary to demonstrate a reliable cost-benefit for erythropoietin use in elective surgery with minimal to moderate risk. Most recently, the use of hemoglobin-based oxygen-carrying substitutes is being explored. Their benefits include a prolonged shelf life, decreased immunogenic potential, lack of risk for disease transmission, and a potential for greater oxygen carrying capacity.45 Continued investigation will be necessary to delineate the benefit of these products.
Nutritional Status Issues
Nutritional status is becoming an important determinant of overall success during bone reconstruction procedures. Patients presenting with malnutrition must be identified preoperatively through clinical assessment and appropriate serum laboratory testing. Some studies have noted that 20% to 67% of head and neck cancer patients (many of whom may require bone reconstruction procedures with the use of free tissue transfers) are malnourished based on a number of variables at the time of surgical intervention.46 Patients with a recent weight loss of greater than 10% prior to surgery are prone to major postoperative complications including wound infection, fistula, respiratory complications, myocardial injury, and even progression to sepsis.46,47 Albumin, prealbumin, preoperative CRP levels, and BMI have all been described as useful determinants of outcome.48–52 Those patients with low albumin and prealbumin are more likely to proceed with slower postoperative recovery and delayed soft and hard tissue healing; however, these markers can be affected by renal and hepatic dysfunction as well as acute inflammation and should be used in the context of the patients overall status.53–56 Conversely, providing patients with protein nutrition preoperatively can decrease hospital stay and improve bone healing as evidenced in the general surgery and orthopedic populations.57,58 In the postoperative setting, many bone graft and bone flap procedures require intraoral incisions. There is substantial debate and no consensus regarding the use of oral rest postoperatively. Patients do require adequate nutrition for wound healing. It is well documented that early nutrition in the postoperative period equates to better patient outcomes, which is especially true of larger flap reconstructions in a critical care setting.59,60 In any circumstance, four options are available. First, a period of oral rest for up to a week is sometimes advocated for gastrointestinal procedures but has never openly been advocated for maxillofacial surgery wound healing. It is generally felt that particulate food can harbor bacteria and predispose to infection and wound breakdown if it is not being cleared adequately. For large reconstructions or difficult wounds, enteral feeding tubes may offer the benefit of nutrition by bypassing the oral wound. While the oral tissues are rested, the patient can still achieve appropriate nutritional support. Complications from nasogastric tube placement have been reported, including intracranial placement in trauma patients, intrapulmonary feeding, tracheoesophageal fistula, sinusitis, diarrhea from dumping syndrome, and gastric ulcerations among other problems. For certain cancer reconstructions, a prolonged oral rehabilitation may be encountered and selected patients may benefit from a percutaneous or open gastrostomy tube. Major complications may include peritoneal placement, colon or intestinal perforation, peristomal infection and granulation, and other feed-related problems.61–64 Some have felt that parenteral nutrition has its advantages in specific populations, but it is associated with a higher rate of sepsis and intestinal atrophy, most notably in a critical care setting.59,62,65
Effects of Diabetes Mellitus and the Use of Corticosteroids
Poorly controlled diabetes mellitus as well as perioperative corticosteroid use are shown to decrease bone and tissue healing. High glucose concentrations, as well as prolonged corticosteroid use, inhibit collagen cross-linking, which is necessary for proper bone and soft tissue healing.66 Additional inhibitive properties on angiogenesis, macrophage and neutrophil chemotaxis, migration, and phagocytosis are also exhibited.67–69 Bone healing is impaired with alterations in osteoblastic and osteoclastic function as well as vascular ingrowth and remodeling.69–75 Poor glucose control is a known risk factor for bone graft site dehiscence and subsequent failure.76 Glucocorticoid therapy is commonly used in the perioperative period with minimal effect on wound healing and infection rates when given over short courses, and without excess dosing. Prolonged corticosteroid use, though, inhibits collagen synthesis and cross-linking, decreases osteoblast and osteoclast differentiation and function, and thus impacts healing.77–80
Nicotine Effects
Nicotine has been shown to have substantial effects on the vascularity of all tissues. By eliciting microvascular vasoconstriction, promoting hypercoagulation with increased fibrinogen levels and platelet aggregation, and creating volatile agents and free radicals, an inflammatory environment is produced that limits healing. Even in low doses, nicotine has been shown to have deleterious effects on bone healing with suppression of pro-osteogenic bone morphogenetic proteins.81–83 Smoking should optimally be stopped well in advance of elective bone reconstructive procedures to maximize bone regenerative capacity. Smoking during the postoperative healing phase has been shown to be detrimental to bone graft survival,84–86 and smokers may have twice the rate of complications as nonsmokers in monocortical onlay grafts.87
The Influence of Active Infections
In rare circumstances, patients may have an underlying infection or severe inflammation to the recipient bed or at the donor site. It is imperative that formal infections be properly evaluated clinically prior to proceeding with any grafting procedure as risks for potential failure or adverse outcomes increase.88 A vascularized flap does offer some protection to mild inflammation at the recipient site; however, prudence is imperative to minimize graft loss and failure. Appropriate patient selection is essential and use of reconstructive procedures in infected sites is best avoided when possible.
DONOR BONE SELECTION CONSIDERATIONS
Autogenous Bone Grafts
Autogenous bone is the current gold standard of hard tissue transfer, and both bone grafts and bone flaps can be utilized to reconstruct ablative, traumatic, infective, and congenital defects. Autogenous grafts carry some viable cells but require ingrowth of adjacent vascular supply (primarily from periosteum and adjacent marrow) to promote successful healing and integration to the native bone. Data suggest that defect shape and size will determine appropriate reconstructive options, as volume and quality of necessary bone can determine the appropriate donor site. Segmental defects of the mandible are among the largest and most difficult grafting procedures performed in the maxillofacial region due to geometry, chin projection, and curved shape. Complications are commonly related to infection, dehiscence, or nonunion. Postoperative wound dehiscence is the most common complication that can result in loss of a portion or all of the graft.89–92 While infection among both vascularized and nonvascularized groups range between 8% and 10%, overall complication rates for nonvascularized bone grafts may be as high as 69%. In a retrospective review of 47 maxillary reconstructions, Smolka and Lizuka93 compared the outcomes and complications of nonvascularized bone grafts, microvascular soft tissue flaps combined with free bone grafts, and composite osteocutaneous microvascular flaps. Postoperative infection occurred in over half the cases of free bone grafts wrapped in vascularized soft tissue, while only 8% of osteocutaneous free flaps developed infection. Complete graft loss was noted in 25% and 16% of free bone grafts and osteocutaneous free flaps, respectively, mainly due to infection. Other complications included wound dehiscence and oroantral/oronasal fistula. One of the largest series of nonvascularized iliac crest bone grafts for segmental mandibular defects in 74 patients was reviewed by van Gemert et al.94 While 76% of patients ultimately had a successful outcome, 43% of patients had postoperative complications. These complications were significantly associated with the site of the defect and the presence of intraoral communication. The authors concluded that nonvascularized iliac bone grafts for the mandible are best used for lateral defects and only with an extraoral approach. Pogrel has published that segmental mandibular defects less than 6 cm portray only a 20–25% complication rate with use of nonvascularized cortical grafts. When grafts greater than 6 cm are used the risk of failure increases significantly. Although a successful bony union rate of 40% was demonstrated for defects up to 14 cm, vascularized bone offers a more predictable and viable option and should be considered when possible.95,96 A determination of morbidity based on defect characteristics (including presence of soft tissue coverage), donor site risk, and procedural length must be weighed for defects between 4 and 7 cm. Use of vascularized bone should be limited for defects under 4 cm due to established success with nonvascularized bone, unless a substantial amount of soft tissue is necessary.
For maxillary sinus grafts, postoperative complications are generally related to infection. When a sinus graft is jeopardized by infection, complete removal and healing for 6 weeks may be required to eliminate infection before regrafting.97 Hematomas in the sinus cavity can occur due to the difficulty in controlling bone bleeding.98 Patients with a preoperative history of sinusitis have a higher incidence of postoperative complications after sinus augmentation procedures including infections, dehiscence, and development of oralantral fistulas.99,100 Soft tissue coverage for bone grafting is occasionally necessary and an assessment of appropriate coverage must be made prior to undertaking surgery. While locoregional flaps may be used for soft tissue coverage, they must be accounted for during the initial plan to allow for proper consent for the patient, given the known risks of each soft tissue coverage flap [random pattern mucosal finger flap, facial artery myomucosal (FAMM) flap, palatal rotation flap, anterior- and posterior-based tongue flap, buccal fat pad, temporoparietal galeal flap, superiorly based platysma flap, submental island flap, pectoralis major pedicled flap]. Microvascular soft tissue flaps can be used for coverage with known risks and complications of each flap.
Risks of Allografts and Xenografts
Allografts and xenografts have increasing use given their availability, low cost, lack of donor site morbidity, and sterile shelf-life. Many options are available for use with particulate cancellous, particulate cortical, and corticocancellous sheets being available. There have been some concerns with the risk of disease transmission, but with proper screening procedures and appropriate sterility assurance levels (below 10−6), the chance of organism transmission overall is 1:1 million. Estimations for specific transmission such as HIV range between 1:1.6 million and 1:8 million and substantially lower when using demineralized formulations.101–104 There are a multitude of techniques used to disinfect and sterilize off-the-shelf graft materials. These include physical debridement, ultrasonic washes, antimicrobial therapy, ethanol soaking, ethylene oxide, electron beam irradiation, and gamma irradiation. Based on the type of bone graft and the techniques employed by the distributer, a combination of these are used while trying to maintain beneficial properties of the graft.102–104 Xenografts have their own concerns due to recent media concern over prion transmission and bovine spongiform encephalopathy. While no reports for transmission have occurred in the maxillofacial literature, a theoretical estimated risk has been calculated by groups, based on purification techniques. A worst-case scenario has listed the risk on the order of 1 in 1010.3 correlating to a virtually nonexistent transmission risk based on preparation and animal selection processes.105,106
Bone Morphogenetic Proteins
Recombinant bone morphogenetic protein-2 (rhBMP-2) has shown some promise for bony reconstruction, although the indications are still not completely defined at present. Perhaps the most concerning postoperative sequela associated with this treatment relates to the impressive swelling, which ensues after placement of the graft. The use of rhBMP-2 in cervical spine reconstruction has been associated with significant neck swelling and dysphagia in up to 27% of patients, sometimes resulting in prolonged hospital stay or readmission.107,108 While the relevance for maxillofacial reconstruction is difficult to ascertain, similar concerns have been raised in the use of rhBMP-2 for segmental mandibular defects.109 Another phenomenon unique to rhBMP-2 is ectopic bone formation.110,111 This is likely due to intraoperative spilling of the reconstituted solution onto the surgical field around the defect being treated. The most serious consequence of ectopic bone would be ankylosis of the temoporomandibular joint if used around the condyle but to date this problem has not been reported.
Reconstruction of mandibular continuity defects using rhBMP-2 is currently being explored, although only small case series exist at this time. In 2008, Herford and Boyne reported their technique in 14 patients, which resulted in successful outcomes in all 14 patients.112 Herford later reported on two cases of using rhBMP-2 with demineralized bone in the successful reconstruction of lateral segmental defects.113 These articles both discuss the need for a technique that maintains space for the graft since the sponge carrier is soft and easily compressed under the soft tissue envelope. A separate series demonstrated successful outcomes using BMP-7 for segmental defects of the mandible in seven patients and a large peripheral ostectomy in three others with no reported complications. Others have not been as successful, as evidenced by Carter et al. in their series of five patients described in 2008. While three patients achieved union, two suffered from nonunion due to chronic infection and collapse of the soft tissue envelope resulting in graft loss.114,115 Other complications of rhBMP-2 include hematoma, seroma, and bone resorption at the graft site.111,116
Specific Donor Site Morbidity and Complications
Autogenous bone reconstruction must take into account the possible complications and morbidity of the donor site. Each donor site has inherent advantages, limitations, and potential complications. For very small defects, intraoral sources are ideal. Extraoral donor sites by contrast imply higher morbidity and risk, but do offer greater amounts of reconstructive material.
Intraoral Donor Sites
Common intraoral sources of bone grafts include the mandibular ramus, symphysis, tuberosity, and coronoid process. A comparison of two sites in 50 patients by Misch suggested that the symphysis donor site is associated with more problems in postoperative healing compared to ramus grafts.117 Wound dehiscence occurred in 3 of 28 patients who underwent grafting from the symphysis, while all ramus donor sites healed without wound breakdown. The author noted that only the vestibular incisions suffered from healing problems in the symphysis, while sulcular incisions healed with fewer problems. Postoperative neurosensory changes from intraoral donor sites are related to proximity of the long buccal nerve, inferior alveolar nerve, and mental nerve. Patients seem less likely to notice sensory changes in the buccal nerve distribution as compared to the lower lip.117 Misch reported a 10% incidence of temporary mental nerve paresthesia after symphysis grafts, but all patients eventually recovered. In the same series, 29% of patients with symphysis grafts reported altered sensation to the lower incisors, which lasted up to 6 months. No patients who underwent ramus grafts demonstrated permanent postoperative changes along the inferior alveolar or long buccal nerve distribution, although other authors have reported low rates of temporary neurosensory changes.118 Postoperative infection seems to be uncommon with intraoral donor sites. A 2009 review of 32 patients who underwent ramus grafts found only one site with a localized postoperative infection, which responded well to incision and drainage.118 While not directly reported, coronoid bone grafts have inherent risks of trismus related to injury of the temporalis muscle, injury to the inferior alveolar and lingual nerves, and injury to the masseteric branch of the internal maxillary artery, which can cause profound acute blood loss. This latter risk is minimized by performing the osteotomy from medial to lateral. The tuberosity harvest site yields poor quality and limited quantity of bone that lead to early resorption of the graft. In addition, sinus exposure and associated sequelae or oral antral fistulas or sinus infections may occur. For these reasons the tuberosity is rarely used as a donor site.
Iliac Crest Harvest Site
The iliac crest is one of the most commonly used donor sites to reconstruct moderate to large bony defects. Both the anterior and posterior iliac crests are available for harvest. Gait disturbance and pain in the early postoperative period is considered normal sequelae of surgery that should resolve with time.119–121 Gait disturbance has been attributed to muscle dissection of the gluteus, iliacas, and tensor fascia lata, particularly when block grafts are required. In contrast, when only cancellous bone is harvested this complication may be limited with careful technique. Although repositioning the patient intraoperatively is required to access the posterior crest, this location has gained the reputation of having less morbidity from gait disturbance, less pain, and fewer hematomas.120,122 The need for repositioning the patient perioperatively carries its own risks of endotracheal tube displacement or occlusion, eyes or nose injury, and requires special attention to taping and padding. In a prospective study of 50 patients that compared the morbidity between the anterior and posterior approaches for iliac crest bone harvest, the authors preferred the posterior ilium due to the lower severity of pain and gait disturbance.123
Infection at this donor site is uncommon and usually minor. Resolution can often be obtained simply with local measures that may require removal of sutures and drainage through the incision site.121,124,125 Postoperative hematomas may occur in up to 6% of cases and are generally minor as well.121,122,126 Most are the result of blood oozing from the marrow, although the deep circumflex iliac artery and muscular perforators may also contribute to bleeding.119 While some surgeons recommend the placement of drains,119,127 this can usually be avoided with use of bone wax, oxidized cellulose, activated thrombin–gelatin matrix (Floseal®, Baxter, www.baxter.com; Surgiflo®, Ethicon, www.ethicon360.com ), and meticulous hemostasis of the soft tissues. An exceedingly rare complication of retroperitoneal bleeding with patient death has been reported.128 Hematoma formation in the posterior ilium may be minimized by postoperative bed rest in the supine position the first night after surgery. Seromas have been reported commonly as a complication and may be treated with aspiration and pressure dressing. If these conservative measures fail, seromas and hematomas may require a return to the operating room for formal evacuation.129 Fractures of the iliac crest may occur after harvest of the anterior or posterior ilium. While this fracture may occur intraoperatively, postoperative fractures have been described secondary to sudden contraction of the lateral musculature along a weakened iliac crest.130 Pelvic instability may also occur after posterior iliac crest harvest and is due to weakening of the sacroiliac crest ligaments.131 Removal of a full thickness segment of anterior ilium has been associated with postoperative contour deformities of the hip.126,132,133 This may be prevented by leaving the crest intact while harvesting bone only from the medial table when possible. Alternatively, trephination allows removal of deep cores of bone while maintaining the overall integrity of the ilium.
Postoperative paresthesia has been reported most commonly in the distribution of the lateral femoral cutaneous nerve (0–17%).121,125,134,135 Injury to this nerve may be minimized by avoiding excessive traction and preserving 1 cm of bone at the anterior superior iliac spine. Other nerves at risk include the ilioinguinal, iliohypogastric, cluneal, sciatic, and subcostal nerves. A postoperative hernia may develop (0–0.8%) with poor reapproximation of muscular and fascial landmarks.135–137 Postoperative ileus is considered extremely rare and has been described in a case report of two patients.138 Careful dissection with protection of the periosteum along with detailed knowledge of the local anatomy should assist in avoidance of severe complications that usually are due to loss of orientation and aggressiveness.
Calvarial Bone Grafts
The calvarium is commonly used as a corticocancellous bone source for maxillofacial reconstruction. The donor site is easily included in the surgical field, and minimal dissection is required to reach bone. Grafts can be easily and quickly harvested from the calvarium for a wide range of purposes including nasal reconstruction, orbital reconstruction, and mandibular onlays, to name a few. While a fullthickness graft is possible, harvesting only the outer table of bone is generally the safest approach. Postoperative hematoma and seroma are the most common complications associated with calverial bone graft harvest and minimal pain has been reported.139 Avoidance of hematoma may be minimized by ensuring hemostasis with bone wax or a scalp head wrap dressing. A review of 586 calvarial bone grafts observed five seromas and two intracranial hematomas for a 1% overall complication rate.140 This report also highlights the potentially serious complication from calvarial bone harvesting in which inadvertent perforation of the inner cortical plate with dural tear or direct cerebral cortex injury can ensue. Neurologic complications, including postoperative hemiparesis, were reported although these were ultimately found to be temporary. Overall, inner table violation has been reported in the range of 0 to 13% resulting variably in subdural hematoma, cerebrospinal fluid leak, central nervous system infection, and sagittal sinus penetration. A number of surgical considerations are important to avoid this complication. First, the surgeon should consider the location of the graft carefully. Some have advocated grafting from the nondominant hemisphere to limit the extent of injury should complications occur. In addition, regardless of side, the overall thickness of calverial bone, which is variably based on the location of harvest, needs to be considered. The thickest and most desirable for grafting in the adult is generally located high on the parietal bone but at least 2 cm lateral to the midline to avoid the region of the sagittal sinus. Preoperative imaging with computed tomography (CT) scanning has been advocated by some surgeons but would generally not be required in the adult population. In children, cranial bone split procedures are generally not undertaken prior to 3 years of age, and at that point a CT scan to assure a diploic space may be warranted. At around 9 years of age, the parietal bone reaches a thickness of approximately 6 mm. Surgical techniques have been variably reported. For outer cortex grafts, carefully outlining the donor site to diploic bone followed by judicious beveling of a least one edge will allow for appropriate angulation of the osteotome for cortical separation. Surgeon preference regarding osteotomy size, shape, and thickness is likely secondary to meticulous technique.139–143 Dural exposure without a tear is generally not a serious event, but dural coverage with pericranium or off-the-shelf dural substitutes is warranted as well as extended spectrum antibiotic coverage. Consideration for neurosurgical input is warranted, particularly when the complication results in dural tear requiring repair or frank injury to the intracranial contents.140
Postoperative donor site infections tend to be superficial and may resolve with drainage.144 Progression to central nervous system infection is rare but may have devastating results. Delayed wound healing of the scalp is uncommon and was reported in only two cases of a series of 247 cranial bone harvests over a 6-year period.142 Postoperative contour deformities of the skull tend to be minimal, although patients may be bothered when the defect is palpable.142,145 These defects may be minimized by generous beveling of the defect edges after graft harvest.140,146 Alternatively, the surgeon may place cranial bone shavings in the defect to augment the contour.142
Alopecia, scarring, or keloid formation along the incision line can occur, and there may be significant cosmetic complications associated with cranial bone graft harvest especially when these sequelae are visible. Care not to transect hair follicles, avoiding the use of hemostatic clips and electrocautery on the scalp flap, and placement of the incision with consideration to the hair receding along hair line, coupled with meticulous wound closure, will assist in minimizing occurrence of these potential complications.
Costochondral Grafts
Costochondral grafts are frequently employed for bony reconstruction of the face particularly when both bone and cartilage are needed. Postoperative complications include atelectasis, pneumonia, pneumothorax, and wound infection. Normal postoperative pain leads to splinting of the chest wall by the patient to decrease movement and further pain. An additional consideration for this pain occurs when the graft is harvested is from the left chest where pain can mimic the findings of acute chest pain from myocardial infarction. Surgeons should consider this carefully particularly in patients with higher risk for cardiac complications. This reduced inspiratory effort can promote atelectasis and pneumonia. A series of 300 patients who underwent rib harvesting noted pneumonia was the most common complication (eight patients) while persistent atelectasis occurred in two patients.147 Postoperative respiratory difficulty is increased as the number of harvested ribs increases.148 Since pain control plays a vital role in the postoperative respiratory recovery of these patients, the surgeon may consider an intercostal nerve block with a long-acting local anesthetic at the termination of the procedure.149 Aggressive use of incentive spirometry and chest physiotherapy should be employed to minimize risk of postoperative respiratory sequelae that could progress to pneumonia.
Intraoperative pneumothorax is generally avoided with wide access and precise surgical technique. The complication is increased when multiple ribs are harvested in a single setting or when large portions of cartilage are removed. As an average, this complication occurs in approximately 5% of patients (Fig. 13.1). Care should be taken particularly when unwrapping the periosteum from the deep portions of the rib and when working in the area of the cartilage to avoid damage to the underlying tissues. Careful inspection of the surgical site should be undertaken after harvest is completed with underwater examination of the wound while the anesthesiologist applies positive pressure. Notation of any clinical tear or bubbles on positive pressure is indicative of pleural tear. Treatment of pleural tear depends on the size and extent of injury. Repair may be attempted over a small Foley or red Robinson catheter with evacuation of pleural air underwater prior to final closure. Muscle patches for larger tears may be helpful, and ultimately a decision for chest tube placement may need to be made. In addition, it should be recognized that a delayed pneumothorax may occur if sharp edges of the remaining cut ribs lacerates the pleura during respiration.149 These remaining ends of cut ribs should be inspected and smoothed if necessary prior to closure. Regardless of intraoperative perception, a postoperative radiograph for evaluation of pneumothorax is indicated given that pneumothorax can be present despite the lack of corroborating intraoperative findings. Treatment of pneumothorax is dependent on size, symptoms, and patient-related factors.
Fig. 13.1. Right pneumothorax following rib harvest.
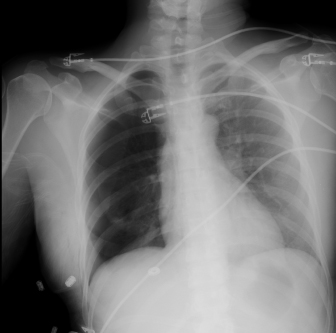
Wound infections following rib harvest are fortunately rare and occur in fewer than 3% of cases.132,150–152 Long-term pleuritic pain has been described132 and may be attributed to scar formation that tethers the pleura to the chest wall. Chest scars occasionally become widened133 and often heal with suture tracks.132 These deformities may be minimized by placing adequate deep dermal sutures,149 removing skin sutures early, and by placing incisions in lines of minimal tension such as the inframammary crease.
Intraoperative and postoperative complications at the recipient site with costochondral grafts deserve consideration. The current role of costochondral grafts tends to be in condylar reconstruction where rib and cartilage are used. Placement of the appropriate amount of cartilage has been debated, particularly in the growing child where some have suggested that the resulting mandibular growth potential, including the risk for excessive growth, is determined by the cartilaginous component. Though not studied prospectively, a 3-mm cartilaginous cap has generally been accepted to be adequate for reconstruction without risking the potential for excess growth or its accidental severance from the rib perioperatively. To avoid this latter complication, some have advocated maintaining a cuff of periosteum and pericondrium at the junction of these tissues. This technique may add to the durability of the costochondral junction but does increase the risk of pneumothorax.
Additional complications arise in the placement of the rib into the glenoid fossa and its ability to remain in place during the postoperative period. Intermaxillary fixation is employed to assist in joint stability during the healing process. In addition, some have described the use of wires or nonresorbable sutures suspended from the glenoid fossa or temporal region to the rib providing additional support. Immediate and long-term malocclusion is a complication attributed to intraoperative failure and the innate growth potential or lack thereof of the graft. Overall, patients should be prepared for the potential need of a period of occlusal elastic therapy to assist in train the musculature to the new joint characteristics.
Tibial Bone Grafts
Tibial bone has gained popularity due to the ability to harvest distant bone in an in-office setting with relatively low morbidity. Postoperative complications include delayed wound healing, infection, gait disturbance, fracture, and persistent pain or paresthesia. A review of 230 tibial bone grafts from the orthopedic literature revealed an overall complication rate of 1.3%.153 Delayed wound healing may occur in the range of 0 to 4.5% of cases.153–155 One review noted delayed wound healing in an obese patient who developed a seroma requiring surgical debridement and closure over a suction drain.154 Ecchymosis is commonly reported although it resolves with time.154,156 Pathologic fractures may occur at the donor site in up to 2.7% of cases.153,157 Gait disturbance is usually short-lived and resolves within 10 days,158 although ambulatory difficulty as long as 3 weeks after surgery has been reported.155 Persistent pain at the donor site occurs in up to 5% of cases.153,155 A series of 44 tibial bone grafts noted one patient with persistent postoperative joint pain due to surgical entry into the joint space during harvest, which can be prevented by avoiding bone excavation in the region of the tibial plateau.154 Neurosensory disturbance may occur in up to 7.5% of cases, although the paresthesia tends to resolve within a few weeks.156
Pedicled Bone Flaps
A number of harvest sites are available for bone transfer with a pedicled blood supply. Calvarial bone as described above can be transferred with the blood supply from a temporoparietal fascia pedicled flap into the lateral maxilla, orbit, or lateral mandible region. Transfer of the fascia can allow for a larger bone graft to be harvested from the outer cranium;159 however, the resultant bony and soft tissue defect can be more prominent following the reconstruction. In addition, there are additional risks associated with tempororparietal galeal harvest, such as alopecia, facial nerve injury, and trismus.159–163
A variety of other pedicled bone flaps have been described with similar risks and complications as their free bone graft counterparts. While they may provide a larger volume of bone due to the pedicled periosteal blood supply, their applications can be limited due to the amount of soft tissue bulk provided and the limitations in orientation. Such examples are costochondral rib grafts pedicled on pectoralis major muscle or latissimus dorsi muscle, scapula tip pedicled on latissimus dorsi, and clavicle pedicled on sternocleidomastoid.164,165
ADJUNCTS TO MICROVASCULAR BONE FLAPS
Fibula Myoossesous (Cutaneous) Flap
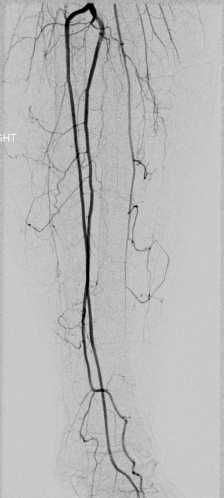
Vascularized fibula bone grafts are often required for reconstruction of larger composite or segmental defects. Preoperative considerations focus on the suitability of harvesting the peroneal vessels while maintaining adequate leg perfusion via the anterior and posterior tibial vessels. Although palpable dorsalis pedis and posterior tibial pulses have been recommended as reliable guides,166 this is no guarantee that sacrifice of the peroneal artery is safe given the possibility of peripheral vascular disease and normal anatomic variants. Kim et al. reviewed 495 lower extremity angiograms and noted hypoplasia or the absence of anterior tibial arteries in 4% of patients.167 Hypoplasia or absence of posterior tibial arteries was noted in 2%. Peroneal arteria magna may occur when both the anterior and posterior tibial arteries are inadequate, resulting in only the peroneal artery supplying blood to the foot. Peroneal arteria magna is estimated to be present in up to 7% of the population (Fig. 13.2).168,169 An absent peroneal artery occurs in 0.1 to 4.0% of the population. Importantly, normal pedal pulses are present in both peroneal arteria magna and with absent peroneal arteries.170 Choice of preoperative lower extremity imaging is often based on surgeon preference and available services in the surgeon’s practice environment. While arteriography is considered by many to be the gold standard, this is an invasive study that carries a 3–5% complication rate. Such problems include contrast allergy, renal failure, hematoma, aortic dissection, and arterial occlusion.168 While the least invasive modality is color flow Doppler imaging, this study is highly technique-sensitive and requires an experienced technician.171 Computed tomography angiography (CTA) and magnetic resonance angiography (MRA) have become standard preoperative modalities in many institutions. MRA has been suggested to be nearly equal to conventional angiography in the preoperative assessment of fibula harvest.170 High sensitivity and positive predictive value have led some authors to utilize this modality routinely in all patients.172 An additional advantage of MRA over conventional angiography is the ability to view the lower extremity vasculature in three dimensions.
Fig. 13.2. Right dominant peroneal artery.
Intraoperative complications in fibula free flap harvest are often related to maintaining the vascular integrity of the skin paddle. While septocutaneous perforators are more easily incorporated into the flap, musculocutaneous perforators are commonly encountered and require inclusion of a muscle cuff around the perforators. Schusterman et al. reported only 33% of skin paddles survived based on a septocutaneous supply, while survival rose to 93% with the inclusion of a muscle cuff.173 Additional complications may occur if the vascular pedicle to the bone is injured. Extreme care should be taken when proximal dissection of the pedicle is undertaken, for injury to the pedicle at this level may render the flap unusable. Distally, aggressive retraction of the osteotomized bone prior to pedicle division may cause separation of the blood supply to the distal bone. To avoid this scenario, some authors recommend removing a small segment of bone at the distal osteotomy.174 This maneuver provides a window through which the pedicle may be accessed and divided prior to lateral mobilization of the fibula.
The fibula donor site occasionally suffers from delayed wound healing, orthopedic complications, contour or cosmetic deformities, and weak or diminished great toe function. Donor site wound healing difficulty is mainly related to the soft tissue component of a composite flap (Fig. 13.3). Incomplete skin graft healing can lead to tendon exposure requiring local wound care for several weeks as healing progresses. A suprafascial dissection under the skin paddle may provide a wound bed more amenable to skin grafting by minimizing the exposure of peroneus longus tendons. Similar wound healing problems may occur if primary closure is attempted after harvesting a skin paddle greater than 3 cm in width. The increased tension along the closure often leads to dehiscence and requires prolonged wound care. The temptation to close the soft tissue defect primarily to avoid a skin graft should be weighed carefully against the possibility of wound breakdown that results in the same cosmetic outcome as a skin graft. Compartment syndrome is fortunately a rare postoperative phenomenon that occurs in less than 1% of cases.175 Orthopedic problems are related to the detachment of muscles from the fibula, injury to the peroneal nerve, or joint instability. The fibula serves to stabilize the ankle joint during function, which requires a 6- to 8-cm distal segment to remain in place. A 10-year follow-up study by Hidalgo176 studied donor site morbidity of 20 patients who underwent fibula free flap harvest. Three of the 20 patients reported intermittent leg weakness or pain and only one was unable to perform rigorous activities such as jogging. One patient in the group was able to run a marathon without difficulty. Postoperative physical therapy should be routinely instituted to minimize functional disturbances.177
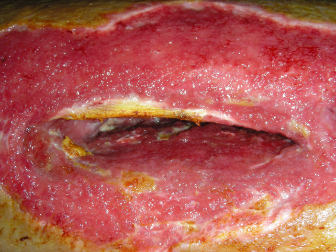
Fig. 13.3. Fibula flap with skin paddle harvest site in a diabetic patient that was closed primarily with wound breakdown and infection treated with debridement and wound dressings.
Deep Circumflex Iliac Artery Myoosseous (Cutaneous) Flap
The iliac crest free flap based on the deep circumflex iliac artery is an excellent source of bone for maxillofacial reconstruction. However, significant donor site morbidity precludes the use of this donor site as a first choice in many institutions. In contrast to nonvascularized corticocancellous grafts, the extent of dissection required for free tissue harvest significantly increases morbidity. Common postoperative problems include ambulatory difficulty, abdominal wall hernia, and chronic pain.178 Hernias may form in up to 12% of patients.179–181 Long-term abdominal wall weakness is most likely to develop into a hernia when a portion of the abdominal wall musculature is harvested. In these cases, a mesh repair of the defect is recommended at the time of harvest.182 Two cases have been reported in the literature describing bowel obstruction due to herniation at the donor site requiring emergent surgery.183 A 2008 review of 24 iliac crest flaps revealed only one donor site hematoma that upon exploration was found to be caused by oozing bone marrow.184 A similar small study found two seromas in 12 patients, which resolved spontaneously.185 No other early postoperative complications were noted in either study. A small number of patients report chronic pain or long-term neurosensory changes. Chronic pain may be related to the use of synthetic mesh for repair of the abdominal wall defect.186 Rare complications reported in the literature include ureteral injury, pelvic instability, and tumor seeding.179
Radial Forearm Osteofasciocutaneous Flap
Preoperative evaluation of the radial forearm donor site must assess the ability of the ulnar circulation to maintain hand viability after sacrifice of the radial artery. Communication between the superficial and deep palmar arches must be present for safe harvest of the flap. This assessment is traditionally performed using the Allen test. The donor radial and ulnar arteries are occluded by the examiner’s thumbs while the hand is exsanguinated by repeated fist clinching. The ulnar artery is released to reveal the extent of hand perfusion while the radial artery remains occluded. Although this test is easily performed at bedside, the assessment is subjective and variable amounts of pressure may be required to reliably occlude the radial artery. Additional error can be introduced if the donor hand is hyperextended. The reliability of the Allen test is well accepted, although some others recommend an objective Allen test using Doppler imaging and photoplethysmography. Nuckols et al. compared the traditional Allen test with an objective Doppler-assisted Allen test in 65 patients and noted an improved ability to detect vascular variations using the Doppler.187 Notably, of the 25 patients found to have equivocal or poor subjective Allen tests, the Doppler exam revealed that 18 of these could safely undergo radial forearm harvest.
The radial forearm free flap is occasionally harvested as an osteocutaneous flap incorporating a segment of the radius. Fracture of the remaining radius is the most feared postoperative complication, occurring in up to 40% of cases.188 Some authors recommend routine prophylactic plating across the radius defect to minimize the chance of fracture.188–190 Others employ postoperative casting of the arm for 6–8 weeks or bone grafting the donor site, although these techniques seem to be utilized infrequently.189 More common donor site problems include decreased pinch and grip strength, reduced sensation over the dorsum of the hand, delayed wound healing, and cosmetic deformities.191–194 A retrospective review of 52 patients who underwent osteocutaneous radial forearm free flap harvest revealed a 7.7% rate of donor site complications.190 These included one radius fracture and three cases of delayed wound healing with exposed tendons. The fracture occurred on postoperative day 3 despite prophylactic plating and was felt to be due to a loose screw. Reoperation and cast immobilization for 4 weeks was required.
Scapula Myoosseous (Cutaneous) Flap
The scapula is a versatile source of vascularized bone that can be tailored to complex defects. Postoperative donor site complications include hematoma, seroma, infection, wound breakdown, shoulder weakness, and chronic pain. While hematoma formation is likely minimized by the normal postoperative bed rest and sleeping supine, seroma formation is common after such extensive dissection of the back. Donor site problems in 36 patients were reviewed that revealed a 25% rate of persistent seroma.195 Little long-term data exist regarding the functional compromise after harvesting the scapula. A 2009 publication reviewed 20 patients who underwent scapula harvest to assess shoulder function 1 and 6 months after surgery.196 Compared with the nondonor arm, the study demonstrated limited mobility in the operated shoulder at 1 month with improvement at 6 months.
BONY RECONSTRUCTION
Distraction Osteogenesis
Distraction osteogenesis has become increasingly common in bony reconstruction as an alternative to standard grafting techniques, with the advantage to generate bone from adjacent sites. Transport disc distraction for segmental defects and alveolar distraction for atrophic ridges are specific examples. Difficulties and complications with distraction have tempered much of the initial enthusiasm with these techniques. While limitations do exist, many of the common complications can be avoided with careful presurgical planning and surgical precision.
The majority of complications relate to the distraction hardware, and the need for exposure through the skin or mucosa. While infection is uncommon, pin-track infections may occur secondary to the open wound, which must be maintained through mucosa or skin during the distraction phase. Because these openings allow drainage, local wound care and irrigation are usually all that are required. Mechanical device failure, instability, or breakage occurs uncommonly and is described as less than 6% for all types of distraction in two large case series.197,198 In two retrospective case series of 37 and 45 patients who underwent alveolar distraction, only one case of a broken distractor was noted.199,200 Other complications were reported as minor (up to 75% of cases), including soft tissue dehiscence (14% to 38%) with infections in 6% to 7%. Major complications included fracture of the basal bone and/or transport segment (8% to 17%).199,200 Another review of 20 patients undergoing alveolar distraction revealed a 55% overall complication rate including fracture of the transport segment in one patient.201 Other reported complications include paresthesia (14% to 28%), hematomas (4%), and postoperative bone defects at the site of the distractor.199,200 Transport distraction is not as widely used with free flap techniques being at the forefront of treatment protocols; however, one series presented 28 patients of maxillary, mandibular, and skull defects with a failure rate of 21% through a variety of causes, including three patients (10%) who died of disease prior to completion of distraction: one patient had device failure with screw loosening on two occasions in an irradiated site, one patient developed early consolidation, and one patient with fulminant infection. Defects up to 80 mm were reported as being successfully distracted.202 Other authors report small case series with custom fabricated distraction devices.203 Additional complications arise when distraction does not result in a bony matrix adequate for healing. This is of particular importance in patients following radiation treatment, where some believe distraction may be of limited use and bone quality is poor; however, a small case series of six patients has shown beneficial results with only one failure (17%). There was soft tissue dehiscence over the distractor that was successfully treated conservatively in two patients.204 Further evaluation of larger case series with both transport and alveolar distraction methods is needed as there is substantial variability of success comparing maxilla to mandible and anterior to posterior with supraperiosteal and subperiosteal methods used.
The most frequent complication for larger defects results from the inability to position the final bone matrix in the desired location. This complication may result from hardware failure itself or more commonly from the surgeon’s inability to appropriately place the distraction device consistent with the desired vectors.197,198,202,205–207 In order to overcome this limitation surgeons have employed computer modeling for accuracy208 and devices that can be adjusted mid-distraction.209 Computer-assisted surgery with surgical planning and modeling allows for the creation of templates, which will guide distractor placement in the operating room.208,210,211 Still, care must be taken to assure that the templates accurately reflect the anatomy since many do not “lock in” to a single position. In addition, the bulky nature of the templates often requires additional surgical exposure, which should be weighed when considering their use.
Hyperbaric Oxygen
Hyperbaric oxygen therapy (HBO) has been recommended by some authors212–217 in the treatment of osteoradionecrosis (ORN) in conjunction with surgical reconstruction. While the initial data seemed promising, the utility of HBO has been challenged by recent more strenuous scientific investigations.218 Conceptually, it seems plausible that HBO therapy would promote angiogenesis and revascularization; however, Annane’s randomized controlled tria/>
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


