13 Bonding to Non-Conventional Surfaces

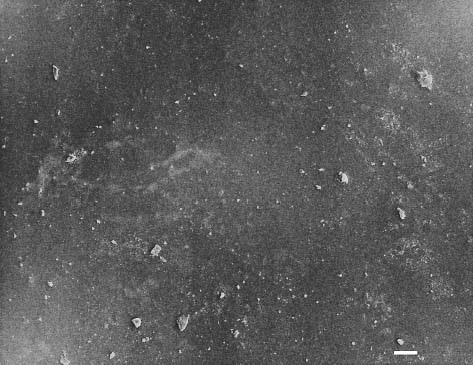
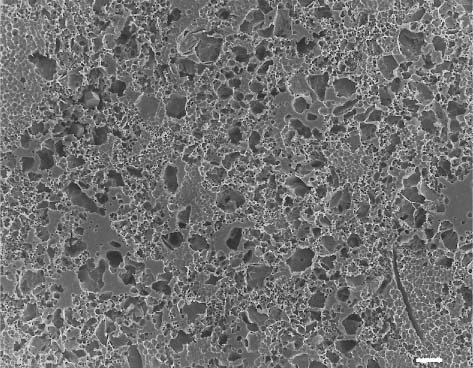
Secondary electron image (SEM) of dental porcelain after grinding the glazed surface with a medium-roughness diamond bur, using a high-speed handpiece. Scale bar length = 10 μm. The right side of the photomicrograph is a magnification of the rectangular area on the left side
Introduction
The scope of orthodontics has expanded over the past two decades to include more adult patients, and it is expected that many of these people will have restorations placed on their teeth. Although banding is always an alternative for the teeth that have restorations, bonding is desirable in aesthetic areas. Additionally, bands tend to accumulate more plaque than do brackets (Chapter 6).
The materials that are in use in restorative dentistry and prosthodontics include ceramics, casting alloys, resin composites, dental amalgams, and acrylic resins. The goal during orthodontic treatment is that the appliances stay securely attached to their original position until the end of treatment. However, once treatment has been completed, the appliances should be easily removed without causing any permanent damage to tooth structure or permanent restorations. This chapter reviews the materials and techniques used for bonding to surfaces of restorative materials, termed non-conventional surfaces in contrast to the conventional bonding to tooth enamel discussed in Chapter 5.
Bonding to Ceramics
Ceramics are used in restorative dentistry as metal-ceramic restorations where the ceramic is bonded to a metal substrate or as all-ceramic restorations in the form of crowns and laminate veneers (Fig. 13.1). The majority of ceramic restorations are metal-ceramic crowns, where dental porcelain is manually formed in several layers (opaque porcelain, body or dentin porcelain, and a glaze) that are fired to a cast alloy substrate. The alloy is generally oxidized before firing of the opaque porcelain in order to achieve chemical bonding at the metal-ceramic interface, although the initial oxidation step is not required for at least one important commercial alloy. The composition of the dental porcelain corresponds to potash feldspar (K2O·Al2O3·6 SiO2) or soda feldspar (Na2O·Al2O3·6 SiO2), or a combination of both, in a silica (SiO2) network that contains leucite crystals (K2O·Al2O3·4 SiO2). During the firing cycles, the porcelain develops a principally vitreous (glass) silicate structure (Chapter 1). Several other ions are added as oxides to the dental porcelain composition for control of color, opacity, sintering temperature, thermal contraction coefficient, and solubility. The microstructure of dental porcelain typically contains crystalline particles of leucite, opacifying oxides, and reinforcing oxides (principally alumina) in a glass matrix.
The all-ceramic restorations can be formed manually and then fired in a manner similar to dental porcelain, or cast in a manner similar to that for dental alloys. The all-ceramic restorations may or may not be core-reinforced with alumina, magnesia, or spinel. It will be very difficult for the orthodontist to determine the type and composition of a given all-ceramic restoration from clinical examination. What is important for the orthodontist is the external surface of the restoration; therefore, this chapter will focus on that aspect. The principal veneering material for all-ceramic restorations is leucite-containing feldspar. However, there are other ceramic materials that have been popular in the past, e.g., fluorine mica silicate in the Dicor (Dentsply, York, PA, USA) castable all-ceramic system. What is important about these castable ceramic crowns is that all of their color is achieved from the external application of stains (metallic oxides). However, this system is not widely used in current dental practice. All other ceramic restorations achieve their color from the intrinsic color of the ceramic layers, with minimal application of external stains. The ceramic restorations should always have a glazed or highly polished surface, as shown in Figure 13.2.
Fig. 13.1 General classification system for ceramic restorations
Acid etching with phosphoric acid in the fashion used for enamel bonding (Chapter 5) is ineffective for bonding orthodontic appliances to dental ceramic surfaces, since these ceramics are not attacked by this acid. Several alternative surface preparation techniques have been found to achieve satisfactory results: mechanical roughening with stones and diamonds (Fig.13.3), sandblasting (Fig.13.4), chemical roughening with hydrofluoric acid (Fig.13.5), a combination of sandblasting and chemical roughening with hydrofluoric acid (Fig.13.6), and chemical coupling with the use of silanes.
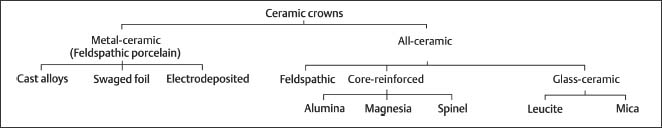
Fig. 13.2 Secondary electron image of a glazed injectable feldspathic dental porcelain (OPC, Jeneric/Pentron, Wallingford, CT, USA) obtained with a scanning electron microscope (SEM). Scale bar length = 10 μm. (Porcelain disks for Figures 13.2 through 13.6 were provided by Dr. Isabelle Denry, College of Dentistry, The Ohio State University)
Fig. 13.3 Secondary electron image (SEM) of the same dental porcelain in Figure 13.2 after grinding the glazed surface with a medium-roughness diamond bur, using a high-speed handpiece. Scale bar length = 10 μm. The right side of the photomicro-graph is a magnification of the rectangular area on the left side
Fig. 13.4 Secondary electron image (SEM) of the feldspathic dental porcelain shown in Figure 13.2, after the glazed surface was sandblasted with a 50 μm alumina powder. Scale bar length = 10 μm
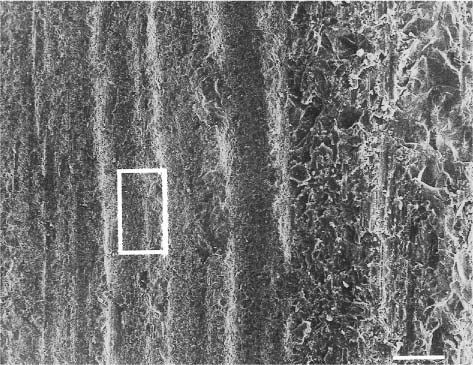
Fig. 13.5 Secondary electron image (SEM) of the same feldspathic dental porcelain shown in Figures 13.3 through 13.6, after the glazed surface was etched with a 9.6% hydrofluoric acid solution for 1 minute. Scale bar length = 10 μm
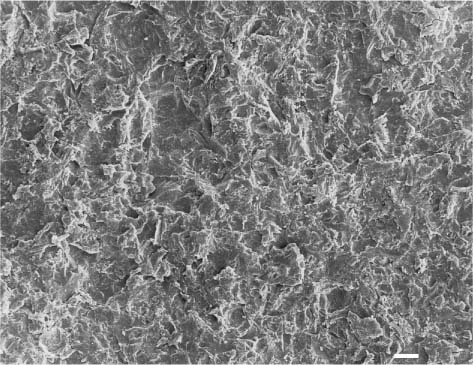
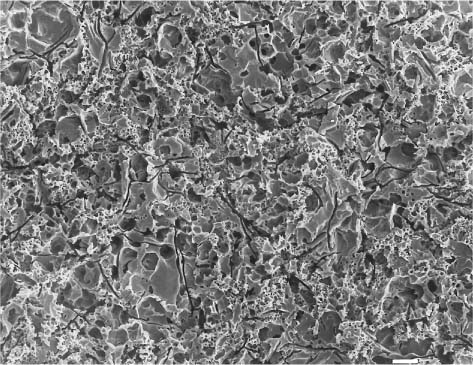
Fig. 13.6 Secondary electron image (SEM) of the same feldspathic dental porcelain shown in Figures 13.3 through 13.5, after the sandblasted surface was etched with a 9.6% hydro-fluoric acid solution for 1 minute. Comparing this figure with Figure 13.5, it can be seen that etching after sandblasting results in a rougher surface than etching alone. Scale bar length = 10 μm
Application of silane to the ceramic surface has been used to promote the adhesion of resin composites. For example, γ-methacryloxypropyltrimethoxysilane is a coupling agent (Chapter 9) that provides reactive sites for inorganic and organic components. This silane contains silanol groups that can bond with silanols on the ceramic surface, forming a siloxane (Si-O-Si) bond. Additionally, this silane contains methacrylate groups that can form covalent bonds with the polymer matrix of the resin composite. There are many different silane products on the market. Some consist of a single component, while others require mixture of two different components. It is very difficult to compare these products, since few have been evaluated in studies, and it is often difficult to compare the results of different studies.
Etching of the ceramic surface with hydro-fluoric acid (HF) was introduced in the early 1980s for bonding porcelain laminate veneers. However, hydrofluoric acid is very potent, and requires considerable care in handling and isolation with a rubber dam. A commonly used hydrofluoric acid product has a concentration of 9.6% in gel form and is placed on the ceramic for two to four minutes (Figs.13.5 and 13.6). Other available commercial products use 4% acidulated phosphate fluoride containing 1.43% hydrofluoric acid in gel form for two minutes. For areas of the ceramic surface where isolation is difficult, a 1.23% acidulated phosphate fluoride gel can be used for ten minutes. Acid etching of the ceramic surface is recommended when the maximum bond strength is required. When there is no such clinical requirement, micro-etching should be used, followed by conventional phosphoric acid to clean the surface and then placement of a silane coupling agent on the surface. Hydrofluoric acid etching without roughening of the ceramic surface does produce sufficient microporosity to achieve successful bonding in some cases.
Upon completion of orthodontic treatment, the ceramic surface should be restored as closely to the original condition as possible. Silicon carbide rubber points of sequential roughness levels and diamond polishing paste can be used to restore the original ceramic surface finish. Many manufacturers provide porcelain polishing kits. However, use of the previously described techniques to increase the retention of orthodontic attachments may create irreversible damage to a ceramic restoration, especially when shade matching was achieved with external staining. The material that provided the original surface staining is removed during polishing, and shade matching with adjacent teeth is possible only with preparation of a new restoration. Additionally, if microcracks or other defects on the ceramic surface have been produced by the use of stones or during debonding, then more ceramic material will need to be removed to achieve a well-polished s/>
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses