CHAPTER 12 MEMBRANE BARRIERS FOR GUIDED TISSUE REGENERATION
In the field of periodontics, the term guided tissue regeneration (GTR) describes an advanced surgical technique used to achieve restitution of the supporting tissues of teeth (i.e., bone, cementum, and periodontal ligament) that have been lost as a consequence of inflammatory disease or trauma.1,2 The technique involves thorough debridement of the bone defect and root surface and then, by means of a cell occlusive membrane, achieving selective cell repopulation of the defect. The protection afforded by the membrane allows the development of slower developing and more complex tissues derived from osteoprogenitor cells and the periodontal ligament, tissues that otherwise may be replaced with gingival epithelium or connective tissue.
In the field of implant dentistry, a related concept known as guided bone regeneration refers to the regeneration of bone that may have been lost due to periodontal disease, trauma, or postextraction atrophy, either prior to or concomitant with dental implant placement. Several treatment modalities have been used in an attempt to reach this goal involving the use of a variety of membrane materials with or without the placement of bone grafts or bone substitutes (Figure 12-1).1,3
The concept of guided tissue regeneration in periodontics was proposed by Melcher, who described the biological behavior of different tissues (e.g., gingival epithelium, connective tissue, periodontal ligament, alveolar bone) during wound healing.4,5 According to this concept, cells that have the capability to form bone, cementum, and periodontal ligament must occupy the defect at the appropriate time and in the proper sequence to result in regeneration of the tissues as opposed to simple repair of the defect. Because the desired progenitor cells reside in the periodontal ligament or alveolar bone, the placement of a physical barrier between the gingival flap and the defect before flap repositioning and suturing was proposed to prevent gingival epithelium and connective tissue (undesirable and faster growing cells) from occupying the space under the barrier.6,7
Although the early studies were concerned with the treatment of periodontal defects, membrane techniques were quickly adapted to facilitate prevention of ridge resorption after extraction, augmentation of alveolar ridge defects, improved bone healing around dental implants, and treatment of failing implants.8,9 According to Mellonig and Triplett, membranes may also be used to provide wound coverage, acting as a duplicate surgical flap to provide added stability and protection to the blood clot.10 In addition, membranes may also provide protection and isolation of the blood clot, creating a space under the surgical flap that will act as the scaffold for ingrowth of cells and blood vessels from the base of the bone defect.11
Studies have shown that a series of complex, interrelated factors influence the predictability of regenerative procedures.12,13 In addition to the creation and maintenance of a blood clot–filled space, which provides mechanical stability and isolates the regenerative space from undesirable tissues, membranes may function as a physical barrier to contamination, preventing inflammation as a result of bacterial invasion into the resolving wound complex, and as a means of concentrating growth factors derived from adjacent bone marrow.
Materials Used for Membrane Barrier Techniques
Various membrane materials have been introduced over the years, along with expanded clinical applications and a general increase in use of membrane-based techniques.8 It has been shown that the biological and physical characteristics of the biomaterials used to manufacture membranes can significantly influence barrier function as well as host tissue response.13 Biocompatibility, space-making ability, ability to achieve tissue integration or attachment, and clinical manageability are criteria that must be considered in the design of materials used for regenerative procedures.14 These materials also should be safe, efficient, cost effective, and easy to use. In addition, they must remain intact as a physical barrier with the ability to exclude unwanted cells until regeneration is complete, yet not interfere with the development of newly formed tissue.15,16
In numerous studies to date, the clinical and histological evidence associated with guided bone regeneration has been favorable. The available evidence suggests that successful regeneration can be achieved with a variety of membrane materials, each of which has particular benefits and limitations, and none of which has been found ideal for every clinical situation.13,17,18 Knowledge of the advantages and disadvantages inherent with each material for the application in which it is being used is important for ensuring success.13
Microporous cellulose filters (Millipore filter, Millipore Corp., Bedford, MA) and expanded polytetrafluoroethylene (ePTFE) (Gore-Tex Regenerative Material, W. L. Gore and Associates, Inc., Flagstaff, AZ) were used in the initial preclinical and clinical investigations of guided tissue regeneration. These materials were not originally manufactured for medical use, but were chosen as barrier materials because they were shown to be biocompatible, and their porosity was such that it allowed the passage of biological fluids across the barrier while excluding certain cell types.5
Cellulose Filters
Nyman et al. conducted the initial studies of the use of cellulose filters in primates with the intended goal of excluding connective tissue and gingival epithelium, allowing cells derived from the periodontal ligament to repopulate surgically created periodontal defects.19 In these early studies, the periodontal ligament, cementum, and alveolar bone on the facial aspect of the cuspid teeth was removed, and cellulose filters were placed over the resulting defects. After healing, histological examination of the healed defects demonstrated regeneration of the alveolar bone and new attachment of new cementum with inserting periodontal ligament fibers.
Nyman et al. were the first to use the regenerative approach on human teeth.19 On a mandibular incisor with advanced periodontal disease, debridement, scaling, and root planing were performed after elevation of full-thickness flaps. A cellulose filter was placed, covering the defect and the adjacent alveolar bone. Three months after the initial surgery, histological examination of the treated defect demonstrated regeneration of new cementum with inserting collagen fibers. Although the initial investigation did demonstrate efficacy of cellulose filters for GTR, disadvantages included premature exfoliation of the membrane and, when used with techniques requiring primary closure, the need for a second surgical procedure for their removal.
Expanded Polytetrafluoroethylene Membranes
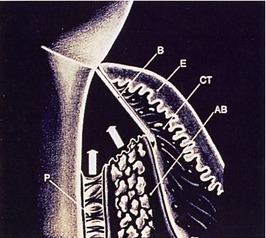
To date, the vast majority of clinical studies involve the use of ePTFE membranes. Due to the early successes and the sheer number of studies and case reports in the literature, ePTFE has been considered the gold standard with which other types of membranes are compared.5,16 Structurally, ePTFE is a microporous matrix consisting of a repeating pattern of nodes and fibrils. By varying the distance between nodes, the material can be made to vary widely in porosity. As a biomaterial with a long history of successful use in vascular surgery, ePTFE is recognized for its inertness and tissue compatibility.20 The size of the porous microstructure may be tailored to allow the ingrowth and attachment of connective tissue for stabilization of the healing wound complex13 (Figure 12-2).
Historically, ePTFE membranes were constructed using both a closed and open microstructure. The open microstructure (collar) portion was designed to facilitate membrane by allowing ingrowth of connective tissue. Theoretically, this ingrowth of connective tissue would have the effect of inhibiting or reducing the chance for epithelial migration between the flap and membrane surface, a phenomenon called contact inhibition. The second part (inner occlusive portion) of the membrane consisted of an occlusive portion that prevented gingival cells from the flap from interfering with the healing process at the defect site.2,5 For periodontal applications, there were two configurations of ePTFE membranes, transgingival and submerged, that could be used in different situations. The transgingival design was used to treat defects associated with structures that extend through the gingiva, such as periodontally diseased teeth. The submerged design was intended to be used in situations in which there was no communication with the oral environment, such as alveolar ridge defects.20
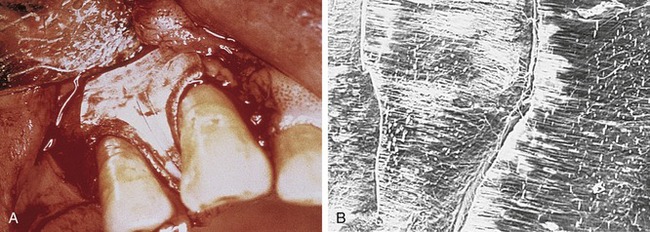
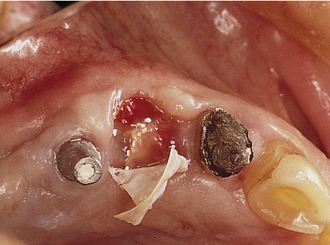
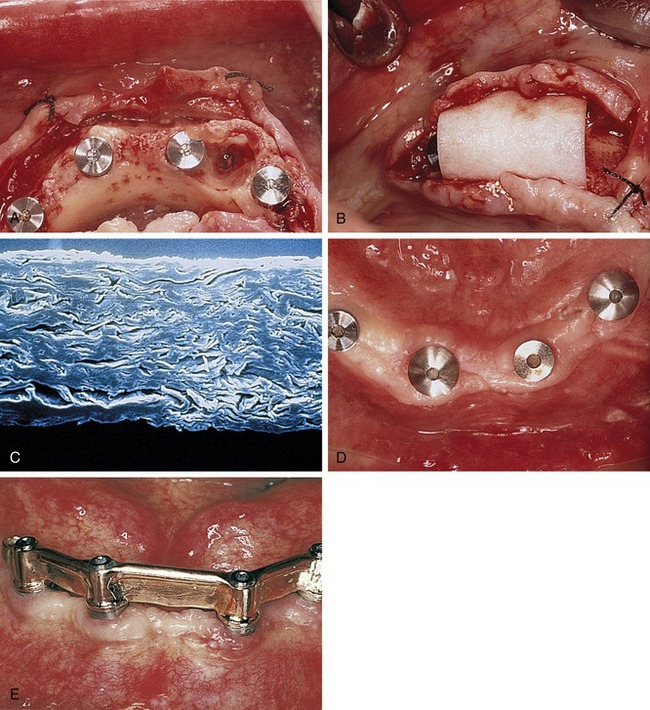
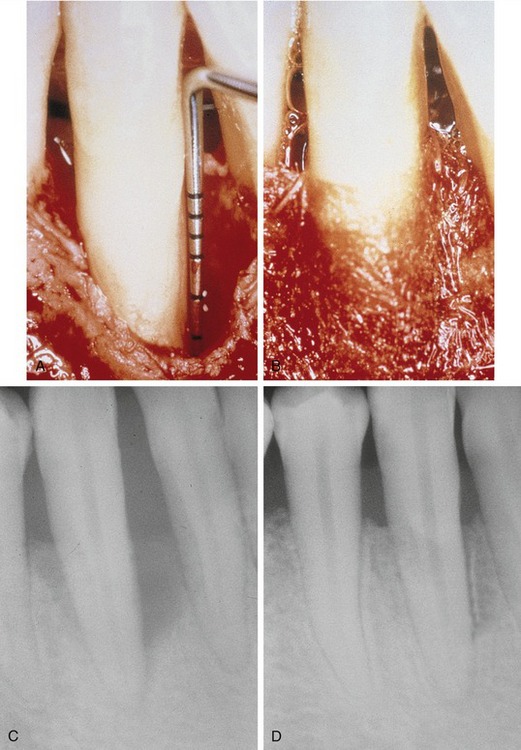
Later, titanium-reinforced ePTFE membranes were introduced, consisting of a thin framework of titanium metal between two pieces of laminated PTFE membrane. Because ePTFE is typically soft and flexible, the titanium reinforcement was designed to increase the space-making capability of the device, creating a tent-like effect over the bone defect. This was found to be advantageous when defect morphology did not inherently lead to the creation of adequate three-dimensional space under the membrane, such as would be found in a typical three-walled periodontal defect. Titanium-reinforced membranes also were available in transgingival and submerged configurations (Figures 12-3 and 12-4).20
Several studies have shown that titanium-reinforced ePTFE membranes have substantial biological potential for regeneration of alveolar bone and periodontal structures. The space created is more predictable and resistant to collapse from overlying mucosal tissue than that created with nonreinforced membranes.21,22
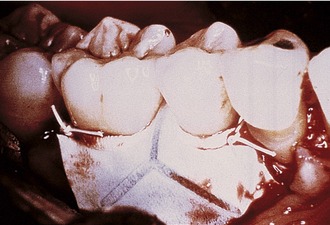
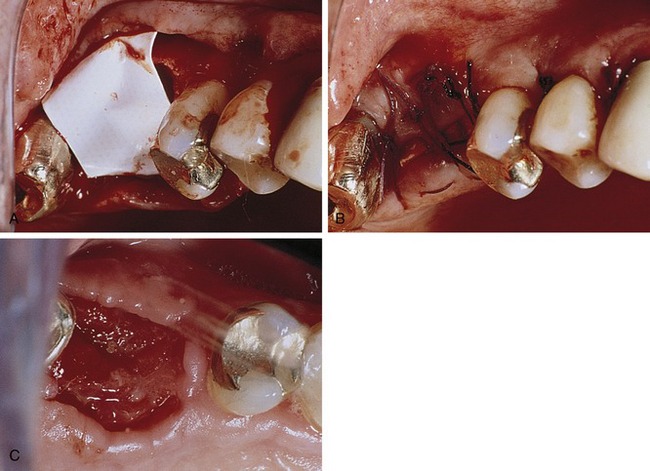
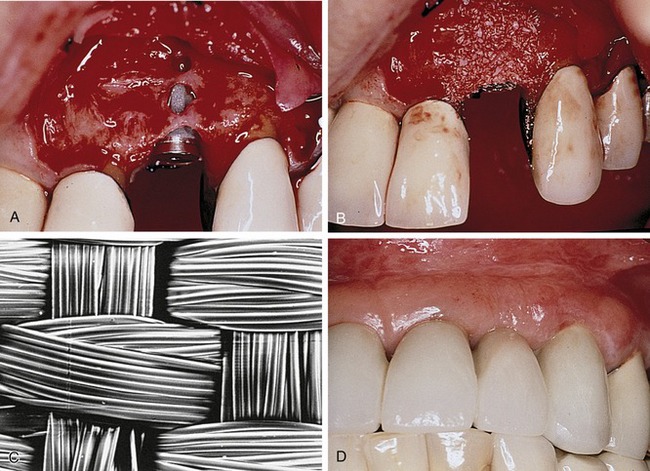
Bartee evaluated and reported on the use of a nonexpanded, high-density PTFE membrane as a guided tissue regeneration barrier.17 Initial reports on this material indicated that the membrane appeared to be well tolerated by the soft tissue, with no inflammation or clinical signs of infection, even when exposed in the oral cavity. The membrane also was shown to provide an effective barrier, allowing bone deposition in the osseous defects. However, the author concluded that more clinical studies are needed to evaluate the effect of this type of membrane. The purported advantage of the high-density PTFE membrane was that it could be left exposed in the oral cavity without the risk of compromising the bone regeneration process, in contrast to the requirement for primary closure with ePTFE (Figures 12-5 and 12-6). This ability to withstand exposure was due to the reduced pore size of dense PTFE, which was designed specifically to prevent the bacterial ingrowth into the dense PTFE membrane structure.
The main disadvantage associated with the use of nonresorbable membranes in general and ePTFE membranes in particular, was that a second surgical procedure was required for removal. There is a major difference, however, in the removal procedure between the two materials. With ePTFE, due to the open microstructure and vigorous tissue ingrowth, surgical removal was often difficult and tedious, increasing the cost and surgical morbidity.5 In contrast, due to much reduced pore size and reduction of tissue ingrowth, removal of high-density PTFE membrane was greatly simplified, and could be accomplished nonsurgically if exposed, and using minimally invasive surgery if primary closure was used over the membrane.
On the positive side, with nonresorbable membranes the clinician remains in control over the length of time that the membrane is in place. It has been suggested that healing times may vary among different types or sizes of defects, especially bony defects of the alveolar ridge.13,22 The principal advantage of nonresorbable membranes, of both ePTFE and high-density PTFE, is that the membrane retains its functional characteristics long enough for adequate healing to occur, and then it can be eliminated immediately at the discretion of the surgeon. After removal, there is no possibility of breakdown products interfering with the maturation of the regenerated tissues as can occur with bioresorbable materials.16
In some situations nonresorbable membranes provide a more predictable performance, with less risk for long-term complications and simplified clinical management.13 Specifically, the use of high-density PTFE membrane may be advantageous in situations in which soft tissue management problems are anticipated and when reliable primary cannot be achieved and maintained. Further, because the removal process is simplified and nontraumatic, it can be accomplished without interfering with the regenerated tissues.16
Bioresorbable Guided Tissue Regeneration Membranes
Following the initial experience with ePTFE membranes for GTR, bioresorbable membranes of polylactide/polyglycolide (PLA/PGA) and collagen were introduced in an effort to reduce the need for additional procedures required for membrane removal.18 However, a disadvantage of bioresorbable materials was quickly realized: premature exposure or flap dehiscence resulting in postoperative tissue management problems. Such exposure in the early healing phase can lead to bacterial growth and premature degradation of the exposed device with loss of barrier function, reducing the success of the regenerative process. Another issue common to the bioresorbable membranes was their inherent mechanical stiffness. Clinicians experienced difficulty in maintaining space under the barriers and preventing membrane collapse into certain types of defects.23
A secondary issue with bioresorbable materials relates to the local biological effects of the resorptive process. To be successful as a guided tissue regeneration barrier, bioresorbable barriers must have similar mechanical characteristics as nonresorbable ones, and the degradation process should not interfere with the regenerative outcome.5,13 The bulk properties of the device and the ratio of PLA to PGA affects the resorption rate and to some extent the local tissue response. PLA/PGA materials degrade via hydrolysis, ultimately breaking down into carbon dioxide and water. High concentrations of these materials’ degradation products (i.e., glycolide) have been shown to stimulate an inflammatory response via complement activation.24–26
Collagen Membranes
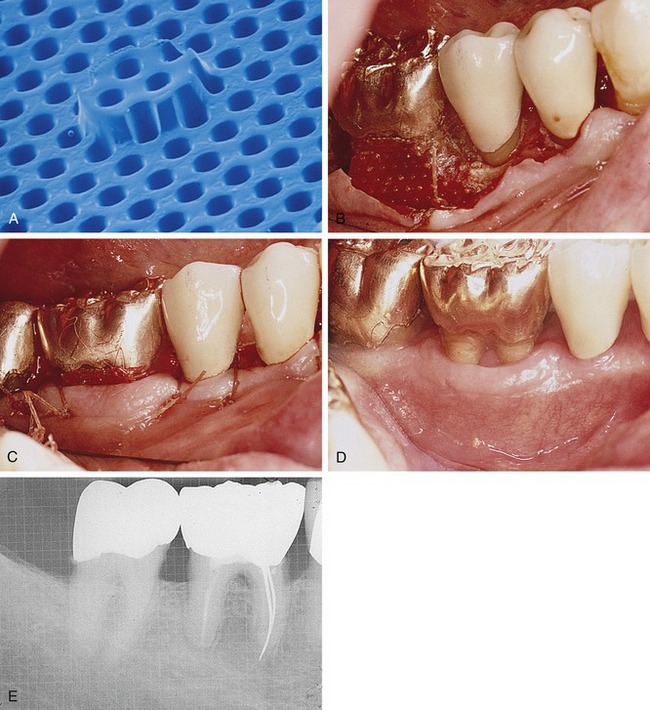
Collagen has been successfully used in various forms (e.g., sheets, gels, tubes, powders, sponges) as an implantable biomaterial for many years.27 As a naturally occurring biomaterial, collagen has a number of characteristics that make it suitable as a barrier material, including high tensile strength and favorable effects on coagulation, cell attraction, attachment, and migration. From a manufacturing standpoint, collagen has additional advantages in that the degradation rate and mechanical properties can be controlled through cross-linking.18,28
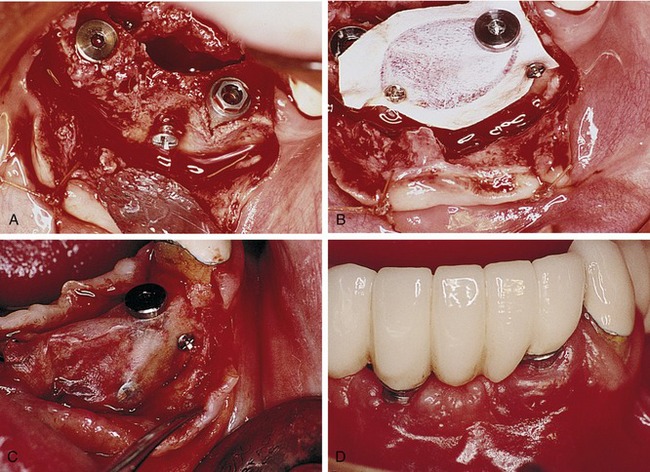
Processed bovine type 1 collagen membranes have been evaluated for membrane barrier procedures in animals and humans with positive results.18 These materials are sourced from a collagen-rich tissue such as Achilles tendon or dermis, hydrolyzed into a gel, freeze-dried, and then compressed into a flat sheet of collagen. Various cross-linking methods are employed, including the use of chemicals such as glutaraldehyde and formaldehyde. Multicenter studies have shown equivalence to nonresorbable membranes in the treatment of periodontal defects (Figure 12-7).18
Pitaru et al. evaluated the degradation kinetics and potential problems associated with premature degradation of collagen membranes.29,30 The authors concluded that rapid degradation (30 days) caused by enzymes found in plaque and healing wounds could result in poor regenerative results. Because of these findings, researchers improved the quality of collagen membranes by using bilayered barriers to compensate for the premature degradation of the external barrier and by adding heparain sulfate and fibronectin to the internal barrier. Fibronectin acted as a chemotactic factor for fibroblasts, binding the heparain sulfate to the collagen membrane. The inner barrier was designed to act as a second barrier for the migrating epithelium and to serve as a delivery system for fibronectin and heparain sulfate. The results of this study showed that the enriched collagen barrier had improved properties to retard apical migration of epithelium compared with nonenriched membranes.31
A multicenter study evaluated the use of bovine tendon type I collagen membranes for membrane barrier procedures in human Class II furcation defects compared the efficacy of bioabsorbable collagen membranes with that of surgical debridement or ePTFE membranes. The results of the study showed that collagen membranes were clinically effective and safe for use in periodontal regenerative procedures. The gain in attachment using collagen membranes was equal to or greater than that obtained with the use of surgical debridement or ePTFE membranes.18
A study by Blumenthal and Steinberg showed successful treatment of one-, two-, and three-wall defects using a collagen membrane combined with antigen-extracted allogeneic bone and collagen gel.32 In this study, a 1- to 2-mm film of collagen gel was placed in the base of the defect, the allogeneic bone was packed into the defect, and a collagen membrane was placed over the defect.
The advantages of using collagen membranes include minimal postoperative complications, minimal antigenicity, rapid healing, and low incidence of dehiscence, tissue perforation, tissue sloughing, or postoperative infection.33 It appears that collagen is a useful and beneficial membrane material for regenerative therapy because these membranes meet the basic criteria for such devices: space maintenance, tissue integration, cell occlusivity, biocompatibility, and clinical manageability.33
Polylactic Acid
A bioresorbable matrix barrier composed of a blend of polylactic acid that was softened with citric acid to improve handling and flexibility was the first resorbable barrier to be approved by the Food and Drug Administration (FDA) as a barrier membrane, but is no longer on the market. This device was a multilayered matrix designed to promote ingrowth of gingival connective tissue and prevent apical downgrowth of gingival epithelium.5 The layer in contact with the bone or tooth (the inner layer) featured small circular perforations and several space holders to ensure enough room for the formation of new attachment, whereas the layer in contact with the gingival tissue (the outer layer) featured larger rectangular perforations to allow rapid ingrowth of gingival tissue into the interspace between the two layers, preventing or minimizing epithelial downgrowth.8,34,35 The resorption profile of the material was reportedly designed to ensure barrier function for a minimum of 6 weeks, after which it was slowly hydrolyzed and metabolized. Complete resorption occurred at approximately 12 months (Figure 12-8).35,36
Several studies have demonstrated the efficacy of polylactic acid membranes in the formation of new attachment and bone in the treatment of interproximal defects and gingival recession in primates, and in infrabony defects and Class II furcation defects in humans.36–39 The results obtained in these studies showed that the use of this matrix barrier around teeth resulted in reduced probing depths, a gain in clinical attachment, and a very low incidence of gingival pathological disease, gingival recession, and device exposure.36
However, Magnusson et al. failed to demonstrate any advantage in the use of polylactic acid membranes in the treatment of circumferential periodontal defects in dogs, contradicting the results of their previous study using the same membranes in dogs.11,40 The reason for the difference in the results may be related to the type of defect: surgically created dehiscence defects on the buccal aspects of maxillary and mandibular premolars versus surgically created circumferential (one-wall vertical and horizontal) defects on maxillary premolars.11,40 A later study by Warrer et al. also failed to show adequate regeneration with the use of polylactic acid membranes (with a nonspecific design) in circumferential periodontal lesions in primates.41 The membrane failed to produce new attachment, and gingival recession and device exposure were common. In addition, an epithelial layer was found in these membranes. These results suggest that the membrane had exfoliated rather than reabsorbed into the tissue. However, the authors concluded that the material should not be considered inapplicable for use in membrane barrier techniques, stating that further modification and transformation were required to create a membrane that possesses all of the properties necessary to obtain better results. Subsequently, the manufacturer removed the membrane from the U.S. market.
Another clinical study in primates compared polylactic acid membranes with polylactic acid mesh barriers. The results demonstrated the superiority of the membranes in the production of new attachment and in biocompatibility compared with the mesh barriers, which showed downgrowth of the epithelium along or around the mesh, gingival recession, device exposure, and pronounced soft tissue inflammation.42 Another clinical study compared the effectiveness of bioresorbable polylactic acid membranes with ePTFE membranes in the treatment of Class II furcation defects in humans. This study showed that although there was a significant gain of clinical attachment with the use of both barriers, there was a significantly greater gain in clinical horizontal attachment and less gingival recession with the use of bioresorbable membranes. Postoperative complications such as swelling and pain occurred more frequently after the use of ePTFE barriers, usually during the first month of healing.43
Roccuzzo et al. compared the reliability of resorbable polylactic acid barriers and nonresorbable ePTFE membranes for root coverage and clinical attachment gain in the treatment of human recession defects and reported no differences for any of the clinical variables assessed.34 However, the advantages of the bioresorbable barrier included less discomfort, stress, and expense because of the single-step procedure. Gottlow et al. showed significantly more new attachment formation and less gingival inflammation and device exposure with the use of polylactic acid membranes when compared with ePTFE membranes.44
Polyglycolic Acid and Polylactic Acid
Bioresorbable membranes made of polyglycolic acid and polylactic acid have been tested in experimental animals and proven to be safe, with a minimal inflammatory response and promotion of periodontal regeneration.16 These membranes consist of an occlusive film with a bonded, randomly oriented fiber matrix located on each surface. The film bonds the fibers and separates the soft tissue from the defect. The random arrangement of the fibers and the openness of the fibrous matrix encourage the ingrowth of connective tissue and inhibit apical migration of the epithelium. The fiber matrix is the primary structural component that provides adequate strength for space-making during the initial phases of healing (two to four weeks for periodontal defects) (Figure 12-9).13
A clinical multicenter study was conducted by Becker et al. to evaluate the capacity of the combination of polyglycolic acid and polylactic acid membranes to promote clinical periodontal regeneration of Class II furcation defects and two- and three-wall infrabony defects.16 After 1 year, the results showed that the defects had healed with favorable changes in the measured clinical parameters (i.e., decrease in probing depths and horizontal probing for the furcations and a gain in attachment levels).
Vuddhakanok et al. studied the use of a biodegradable barrier made of polylactide : polyglycolide (50 : 50 DL-PLGA copolymer) in patients with severe horizontal bone loss and active periodontal disease.45 Historically, this combination has been used for sutures and implant material and in a drug delivery system. Inflammatory tissue response after the implantation of copolymers was found to be minimal, and no adverse host tissue responses were observed. The results of this study showed that the barrier did not enhance connective tissue attachment or prevent epithelial migration. After placement, the material was clinically evident at 10 days to 2 weeks but not after 17 days, indicative of a very rapid resorption rate and inadequate barrier function for guided tissue regeneration.45
A study by Simion et al. compared the use of resorbable membranes made of polyglycolic acid and polylactic acid with ePTFE membranes for membrane barrier procedures. This study showed a significantly greater amount of bone regeneration with the use of ePTFE membranes compared with the resorbable membranes.46 According to the authors, this difference may be because of several factors: (1) the fixation screws may have acted as tent poles to prevent ePTFE membrane collapse, increasing the space for bone regeneration; (2) the stiffness of the resorbable material was not sufficient to maintain adequate space between the defect and the membrane; and (3) as the membrane resorbed, the space-making capability of the barrier decreased.
Synthetic Liquid Polymer (Atrisorb)
A polymer of lactic acid, poly(DL-lactide) (PLA), dissolved in N-methyl-2-pyrrolidone (NMP) as a plasticizer, has been studied as a resorbable barrier material. The material begins as a solution that sets to a firm consistency on contact with water or other aqueous solution.47,48 When outside the oral cavity, the membrane is a partially set solution, which allows it to be trimmed to the dimensions of the defect before intraoral placement. The barrier is then adapted to the defect and sets in a firm consistency in situ. Because of its semirigid property in the extraoral environment, this barrier has the advantage of being rigid enough for placement but flexible enough to be adapted to the defect. The barrier adheres directly to dental structures; therefore, sutures are not required.47,48 Chemically, the material is a polymer component that is resorbed through the process of hydrolysis. The rate of resorption is controlled and the membrane is present during the critical period of healing, preventing epithelial migration and isolating the periodontal defect compartment.48 Alternatively, it can be used by placing graft material in the defect to ensure a tent-like position of the membrane, applying the liquid polymer directly to the surgical site, and then allowing contact with surrounding fluids, which initiates the set-up of the polymer to the firm consistency (Figure 12-10).
Figure 12-10. A well-adapted, well-trimmed customized Atrisorb membrane with minimally adhesive property of the membrane.
Several authors have studied the efficacy of this barrier. Early investigations by Poison et al. in dogs demonstrated that the material is safe, nontoxic, resorbable, and efficiently produces regeneration.49 In addition, the animal model system allowed histological analysis 9 to 12 months after baseline surgery, which showed that formation of new cementum, periodontal ligament, and alveolar bone occurred after the placement of this membrane. Studies in humans also showed the efficacy of this material to produce periodontal regeneration in Class II furcation defects.48 The results obtained in this study were confirmed in a later multicenter study by the same researchers.47
Polyglactin
Another bioresorbable barrier that has been developed as a membrane barrier is a woven mesh barrier made of polyglactin 910, a copolymer of polyglycolic acid and polylactic acid in a 90 : 10 ratio with a resorption rate of 30 to 90 days (Figure 12-11). Several studies have questioned the use of polyglactin for GTR procedures, reporting that the mesh provides an insufficient barrier because of fragmentation of the material. The integrity of the mesh is lost after 14 days, and the cervical sealing between the mesh and the adjacent tooth may not be perfect, allowing for the growth of connective tissue and epithelium between the root surface and the barrier.35,50
A clinical and histological study in primates that compared the design of the mesh barrier with a matrix barrier concluded that the healing process differed considerably, both clinically and histologically. Histologically, complete integration with the surrounding tissue was found with the majority of matrix barriers, preventing epithelial downgrowth and pocket formation around the barrier. However, advanced epithelial downgrowth was found on the mesh barriers. Based on these findings, the author did not recommend the use of mesh barriers for membrane barrier procedures.35 These results were similar to those of previous studies in which epithelial downgrowth, gingival recession, device exposure, and pronounced soft tissue inflammation were observed with the use of mesh barriers.42
Calcium Sulfate
Medical-grade calcium sulfate, commonly known as plaster of Paris, has been used after immediate implant placement as part of a bone graft placed around the implants. Barriers composed of medical-grade calcium sulfate can be placed over bone grafts for clot stabilization and to exclude undesirable tissue (gingival connective tissue and epithelium). This material’s advantages include providing a source of calcium in the early mineralization process and aiding particle retention.51
A study by Maze et al. compared the bone regeneration capability of demineralized freeze-dried bone allograft (DFDBA) in the treatment of mandibular Class II furcation defects. The study compared the capability of DFDBA covered with an ePTFE membrane with DFDBA covered with calcium sulfate.52 They concluded that the results obtained with both barriers were comparable in selected defects. Anson and others showed successful results using medical-grade calcium sulfate and DFDBA for regeneration of periodontal defects.23
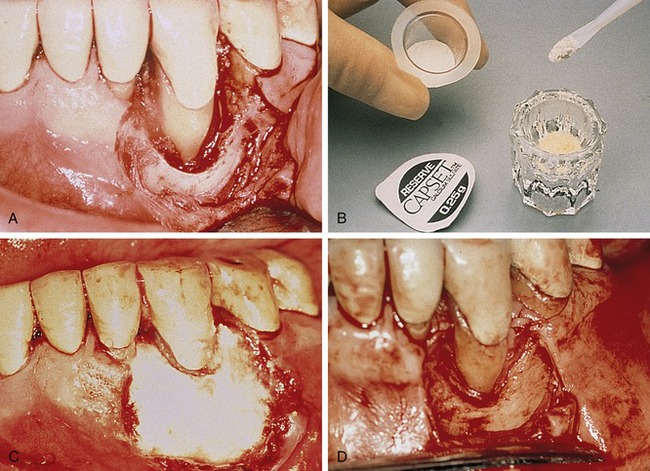
Calcium sulfate has been shown to facilitate complete closure in situations where primary wound closure over the barrier membrane is not possible. An in vitro experiment comparing the ability of human gingival fibroblasts to migrate along a chemotactic gradient over three different forms of membrane barrier materials (e.g., ePTFE, polylactic acid, calcium sulfate) showed that the mean migration distance, as well as cell attachment and spreading, was significantly greater with the calcium sulfate barriers. Based on the results of this study, the authors concluded that calcium sulfate as a membrane appeared to offer greater potential than other membranes for healing by secondary intention in surgical sites where primary closure cannot be obtained.8
This material is available in sterile kits that contain exact amounts of medical-grade calcium sulfate powder and a prefilled syringe of liquid. When mixed together, these substances create a moldable plaster that can conform to the desired shape, even in the presence of blood. Sutures are not required because this mixture is adhesive. Calcium sulfate dissolves in approximately 30 days without an inflammatory reaction, and it does not attract bacteria or support infection (Figure 12-12).51
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses