CHAPTER 9 BONE
PRESENT AND FUTURE
Success in implant dentistry can be directly correlated to the quality and quantity of bone at an implant recipient site.1–6 Bone grafts of one form or another for the management of various osseous defects have been available to surgeons for many years.7–11 Interest in bone reconstruction for the oral cavity has increased dramatically over the past two decades, stimulated by the widespread acceptance of dental implant treatment. Many patients present requesting implant rehabilitation who, in turn, require adjunctive procedures to increase the quantity and/or quality of their recipient bone. Although autogenous bone can be utilized, many patients prefer not to undergo the surgery required for tissue harvesting. The quest for a biomaterial, which does not require the harvesting of tissue and its accompanying time, expense, and morbidity, is significant. Industry continues to present practitioners with new alternative materials.
 Bone
Bone
Bone is a highly specialized mesenchymal connective tissue with a mineralized extracellular matrix that functions to provide support for the human skeleton.12,13 Fossil records date the evolution of bone to the Paleozoic era close to 300 million years ago. Since then bone has evolved to its present state and has established a significant role for itself in vertebrate life forms. Bone possesses a structural function for our bodies, providing form, strength, and rigidity, but it also plays a physiological role.14 It actively participates in maintaining hormonally regulated calcium homeostasis in the body.15,16 Bone is interesting in that it exists both as a skeletal organ and a tissue, which is the predominant substance of the organ.17,18
Embryology
Bone development may be classified as either intramembranous or endochondral on an embryological basis.19,20 When ossification occurs directly, it is defined as intramembranous. Embryonic mesenchymal cells with an abundant vascular supply develop loci of intracellular collagen deposition. Soon osteoblasts can be identified in these regions, secreting osteoid into which calcium salts are deposited. This type of direct bone formation is evident in the genesis of the cranial vault, the facial skeleton, and parts of the mandible, scapula, and clavicle. Endochondral bone formation, on the other hand, involves a cartilaginous phase. Embryonic mesenchymal stem cells differentiate into a primitive hyaline cartilage. Blood vessels and bone forming units, which resorb the cartilage and replace it with osteoid, invade this matrix. Weight-bearing bones and those terminating in joints comprise most of this group. In addition, most of the cranial base and a portion of the mandible are thought to have an endochondral origin.21,22
Cellularity
Regardless of embryonic origin, bone is composed of four cellular types: osteocytes, osteoblasts, osteoclasts, and periosteal bone lining cells.23,24 Osteoblasts are cuboidal cells having a prominent Golgi apparatus and well-developed rough endoplasmic reticulum permitting protein production. These fully differentiated cells secrete the type I collagen and the noncollagenous proteins of bone’s organic matrix. They also regulate the mineralization of this matrix. Osteocytes are thought to be mature osteoblasts that become trapped within the bone matrix. Though their primary function is maintenance, they have demonstrated abilities to synthesize and resorb bone.
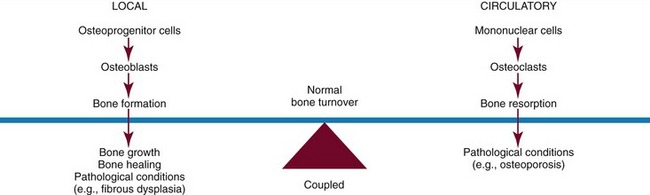
Osteoclasts, unlike the other bone cells that have local origins, arise from the fusion of mononuclear precursor cells originating in the hemopoietic tissues. They function to resorb bone. The cell signaling of osteoclasts involves a complex pathway mediated by the RANK ligand (receptor activator for nuclear factor K B). It is also known as the TRANCE ligand (TNF-related activation-induced cytokine), osteoprogesterin ligand (OPGL), and osteoclast differentiation factor (ODF). The overproduction of RANKL has been implicated in the pathogenesis of a variety of degenerative bone diseases such as osteoporosis and rheumatoid arthritis.25 Osteoclasts are also the cells that are most affected by bisphosphonates.26 Putting all of the delicate elements together, coupling describes a continuous process whereby bone formation and resorption are maintained in balance.27,28 Once this balance is disrupted, excessive osteoclastic activity may lead to problems such as osteoporosis, whereas increased osteoblastic activity may reflect bone growth, healing or pathological responses (Figure 9-1). Severe osteoclastic hypofunction may result in osteopetrosis or bisphosphonate-related osteonecrosis of the jaws.26
Architecture
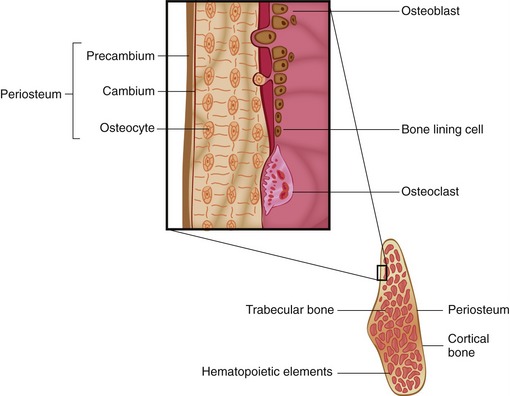
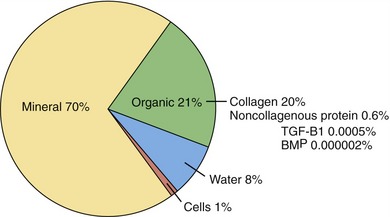
The architecture of bone (Figure 9-2) is such that the outer shell of bone, referred to as cortical (compact) bone, provides the mechanical support. It is composed of concentric sheets of collagen fibrils in the form of lamellar bone. Metabolic functions of bone are controlled by the centrally located cancellous (trabecular/spongy) bone. In contrast to the densely packed fibrils of the cortical bone, the matrix of cancellous bone is loosely organized. Macroscopically, this bone appears as a honeycomb lattice in which hematopoietic elements are located. Bone is composed of 65% to 70% crystalline salts by weight, primarily in the form of hydroxyapatite, with the remaining 30% to 35% being composed of organic matrix. The organic matrix consists primarily of type I collagen (90% to 95%) interspersed with noncollagenous proteins such as osteopontin, osteonectin, bone sialoprotein, and various growth factors (Figure 9-3).29–31
 Bone Formation
Bone Formation
As was noted in the preceding section, the cambium layer is one source of mesenchymal stem cells, osteoprogenitor cells, and osteoblasts, which are necessary for appositional bone growth.32 With the appropriate cell signaling by growth factors, stimulated periosteal mesenchymal stem cells become committed to become osteoprogenitor cells and ultimately differentiate into osteoblasts. Osteoblastic differentiation is characterized by the early expression of alkaline phosphatase and later by expression of the DNA-binding transcription factor Runx-2/Cbfa-1. This Runx-2/Cbfa-1 is responsible for osteoblastic production of collagen,33 the largest constituent of unmineralized osteoid. Mineralization of osteoid forms the new bone, resulting in intramembranous ossification.
Some factors such as bone morphogenetic proteins (BMPs) were originally identified in investigations of embryonic skeletal development.34 They are now known to be involved in signaling during the postnatal period in times of stress or injury.35
Runx-2/Cbfa-1 is a DNA-binding transcription factor that is specific for osteogenic cells. It is one target of transforming growth factor (TGF)-β and BMP, and serves to regulate osteoblast differentiation.36 The stimulation of Runx-2/Cbfa-1 is thought to drive the mesenchymal precursor cell toward osteoblast lineage. Runx-2/Cbfa-1 controls bone formation by regulating expression of all known marker genes expressed by the osteoblast.37
Multiple endocrine factors have been shown to be important in bone formation and skeletal maintenance, including sex steroids, parathyroid hormone (PTH), vitamin A, and 1,25-dihydroxy-vitamin D3. Steroid hormone receptors such as estrogen and androgen receptors in the nucleus of the cell affect osteoblastic differentiation.38 PTH and PTH-related protein are important regulators of osteoblast function. Receptors in osteoblasts signal using the cyclic adenosine monophosphate (cAMP) pathway39 and induce expression of the proto-oncogene cfos during osteoblastic differentiation.40
Vitamin A levels influence bone formation, remodeling, and metabolism. Retinoic acid and BMP signaling are known to cooperate in promoting osteoblastic differentiation.41
1,25-dihydroxy-vitaminD3 enhances osteoblastic production of vascular endothelial growth factor (VEGF). This in turn stimulates endothelial cell VEGF receptor gene expression. The VEGF/VEGF receptor is involved in both formation and remodeling in vivo.42
The family of fibroblast growth factors (FGFs) includes 22 genes that encode structurally related proteins. Specifically, FGF1, FGF2, FGF4, and FGF18 stimulate the growth of fetal calvarial osteoblasts but not mature osteoblasts. FGF2 increases the number of osteogenic cells in vivo and promotes calvarial osteogenesis. FGF and BMP signaling control calvarial growth and differentiation during intramembranous bone development.43 A lack of FGF2 receptor activity is associated with certain craniosynostosis.44
Platelet-derived growth factor (PDGF) is a polypeptide dimer. It was initially isolated from platelets but has been found to be synthesized by a variety of other cells. PDGF is the product of two genes that encode PDGF chains, PDGF-A and PDGF-B, which are mitogenic for osteoprogenitor cells of fetal rat calvarial bone cultures.45
The TGF-β superfamily consists of approximately 30 structurally related dimeric proteins, which include the TGF-βs and BMPs. These proteins can be further classified by the type of receptor-regulated (R)-Smads they activate.46 TGF-β1 stimulates bone formation by chemotactic attraction of osteoblasts and enhanced osteoblastic proliferation. The signaling of TGF-βs is through Runx-2/Cbfa-1, Smads (i.e., Smads 2/3), and Smad-independent pathways to regulate gene transcription.46
BMPs induce osteoblast differentiation in normal human bone with messenger ribonucleic acids (mRNAs) from BMPs 2-9. BMPs 3, 4, 7, and 8 are highly expressed in normal human intramembranous bone.47 BMPs 2, 6, and 9 have also been shown to induce osteoblastic differentiation of mesenchymal cells.47 BMPs signal via three serine/threonine kinase BMP receptors, known as BMPR-IA, BMPR-IB, and BMPR-II.48 Growth factors play important roles in the proliferation and differentiation of osteoprogenitor cells. TGF-β1, FGF-2, and PDGF-AB increase the expression of cell surface BMPR-IB, thereby further enhancing osteoblastic sensitivity to BMP ligands. Once again, Runx-2/Cbfa-1 is required for the targeting of TGF-β and BMP-2-dependent Smads to subnuclear sites.49
VEGF is a protein with a vital role in angiogenesis, vascular permeability,50 and bone morphogenesis.51 VEGF shares homology with PDGF.52 VEGF is a 45 kDa homodimeric glycoprotein with a known chromosome locus at 6p21.3. VEGF signaling is thought to be a rate-limiting step in physiological angiogenesis. VEGF mediates its effects on osteoblasts via osteoblastic VEGF receptors.53 VEGF mediates its effects on primary human osteoblasts via VEGFR-1.53
Hypoxia also affects bone formation.54 Hypoxia acts indirectly on bone repair through the transcription factor hypoxia-inducible factor-1 (HIF-1). HIF-1 is a heterodimer with α and β subunits. The HIF-1α subunit is regulated by hypoxia and undergoes proteosomal degradation under normal conditions. Under hypoxic conditions, HIF-1α is stable.55 HIF-1 directly activates many gene products, including VEGF, VEGFR-1, and erythropoietin.56
 Bone Healing
Bone Healing
Bone is a unique tissue in that it can repair itself after injury and return to full function without scarring or deformity. This phenomenon occurs when certain basic principles are followed. Embryonic bone development is recapitulated in the healing of bone. Dictated by the host bed (vascular supply and oxygen tension) and the stability of the bone segments, healing can occur either directly (primary bone healing) or secondarily, demonstrating an intermediate cartilaginous phase. This same embryonic development is also recapitulated in an even more prolonged manner during distraction osteogenesis.57
Initial Healing
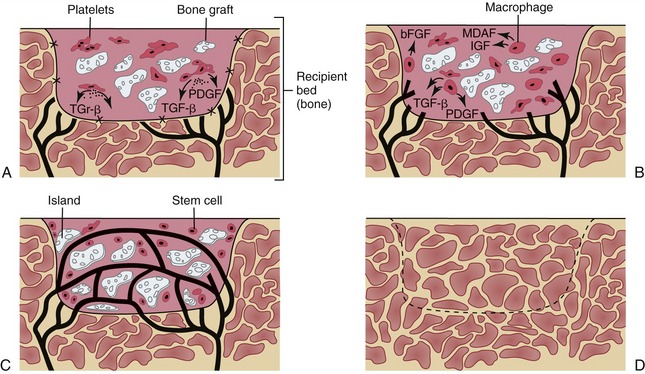
Within hours of placing a graft the initial regenerative process begins (Figure 9-4, A).58–61 Entrapped platelets degranulate, releasing potent growth factors such as platelet-derived growth factors (PDGF) and transforming growth factor-beta I (TGF- β1). Endothelial cells initiate capillary ingrowth as they bind PDGF. Next, endosteal osteoblasts and hematopoiteic stem cells are stimulated to initiate mitosis, increasing their numbers and commencing their production of osteoid. This is mediated by the binding of TGF-ß1 to cell receptors. After the third day, the influence of the growth factors transplanted with the graft is replaced by the action of locally induced macrophages (Figure 9-4, B).62 They efficiently synthesize growth factors and regulate bone healing from this point. By the end of the second week, the graft demonstrates complete revascularization (Figure 9-4, C). Endosteal osteoblasts from the transplanted bone begin laying down osteoid and stem cells begin differentiating into osteoblasts. Resultant islands of bone formation are then seen developing within the graft. Once the graft has become revascularized, circulating stem cells, attracted to the wound, may also transform into bone forming units.63
This intitial bone formation, which occurs as a result of the transfer of osteocompetent cells contained within the graft, has been referred to as Phase I bone.64 Complete by 6 weeks, graft viability is maintained as sufficient quantities of newly mineralized matrix are deposited. Bone formation that has occurred does so without initial cartilaginous deposition and is referred to as woven bone. This bone is extremely cellular and disorganized and does not demonstrate any independent structural integrity. During the second phase of healing, bone undergoes a remodeling phenomenon referred to as lamellar compaction. The resultant lamellar bone is less cellular, more mineralized, and is organized. As with all bone, this newly formed matrix matures as it responds to the physical demands placed upon it. Finally, it enters a remodeling phase similar to normal skeletal turnover (Figure 9-4, D). Some investigators have suggested that, in the end, this grafted bone never develops a cortex that is as thick as native bone, but retains a dense trabecular pattern, which is considered to be good for dental implant placement.
 Bone Regeneration Products
Bone Regeneration Products
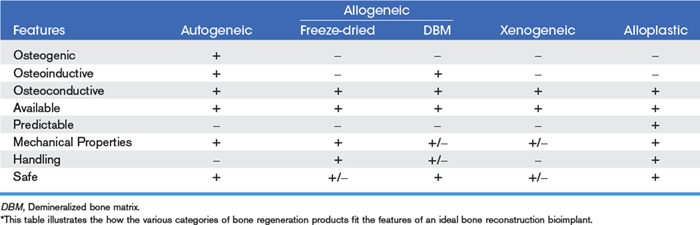
The search for a suitable replacement for autogeneic bone continues to drive the development of new products and the bone regeneration industry.64 New products with exciting claims are being introduced on a monthly basis. The key in making a choice for patients is to separate fact from fiction when it comes to the associated commercial marketing. Bone regeneration can be best examined by identifying the various categories of materials: autogeneic bone, allogeneic bone, xenogeneic bone, and alloplastic bone. Before each group is explored, a list of the ideal properties (Table 9-1) for a bone reconstruction bioimplant should be reviewed.
Autogeneic Bone
Autogeneic bone is the gold standard for bone regenerative grafting materials against which all other bioimplantable materials will be compared. The primary reason for this is its capacity to support osteogenesis in conjunction with its endogenous osteoinductive and osteoconductive properties. In addition, if handled properly it is safe and can be manipulated to assume various mechanical properties depending upon the clinical requirements. The major drawback is that a donor site is required, which can lead to increased time, cost, and morbidity for patients.65,66 For many patients, the graft donor site is more uncomfortable and painful than the actual reconstructive surgical site. In addition, the donor site and its individual variability limit the amount of bone that can be harvested. Studies have shown that, compatible, autogeneic bone may not be of the best quality due to variations in fat content and other related factors.67
Autogeneic bone grafts are usually classified as either vascularized or nonvascularized,67 the difference being the fact that vascularized grafts retain their existing nutrient vessels which, when reattached (anastomosed) at the site of reconstruction will make the graft immediately viable. A major drawback to this form of transplant is that the surgical harvesting and reanastmosing of this type of graft is extremely invasive and creates significant morbidity, which in some cases may be longstanding.65,68 Nonvascularized grafts follow the sequence of incorporation presented earlier in the chapter. There are essentially two forms of nonvascularized free bone grafts: cortical and cancellous.69–72 Cortical grafts are able to withstand mechanical forces sooner after grafting; however, they take more time to revascularize and thus require more time to become vital due to the lack of vascularity. They are useful for filling defects where early mechanical loading is required. The cortical component can be incorporated into the fixation of the graft and can consequently be used in situations in which bone is comminuted or void. In the orofacial complex these forms of grafts may also be used to onlay areas such as decreased vertical or horizontal alveolar ridges or to improve facial contours. Common sites for the harvesting of cortical grafts are the cranial vault, the ribs, and the medial or lateral table of the anterior aspect of the iliac crest as well as the mandibular symphysis.
Cancellous Grafts
Cancellous grafts have more widespread applications, are generally easier to manipulate, and revascularize more rapidly. The most abundant supply is found using an anterior or posterior approach to the iliac crest. It is important to remember that cancellous bone imparts no mechanical strength; when it is used to reconstruct large continuity defects additional rigid fixation is required. In the oral cavity these grafts are used to fill bony defects, alveolar clefts, maxillary sinus, and other similar scenarios where bone can be placed into an area and can be retained. The corticocancellous graft usually produces the best results by combining the attributes of both graft forms.73 It allows for mechanical stabilization while at the same time providing for good revascularization. It is also possible to particulate corticocancellous bone, creating a mixed graft that can be used for the restoration of continuity defects in the jaws. Once again rigid fixation is required, but proponents feel that the improved revascularization and increased Phase I bone make this a very attractive choice for reconstruction.
Allogeneic Bone
Freeze-dried allogeneic bone is processed to remove the moisture from the bone. This results in an implant with mechanical strength that can be used to onlay areas or, more frequently, as a crib or a retainer for autogeneic bone.73 For example, a freeze-dried allogeneic mandible can be very useful for the reconstruction of a continuity defect in the mandible (Figure 9-5). Because the form of the jaw is established in the implant, if the correct size is chosen these normally challenging circumstances can be readily addressed. There has also been considerable interest in bone blocks fabricated using this approach for onlay applications prior to jaw reconstruction.74,75 These implants, while being osteoconductive, have no osteogenic or osteoinductive capabilities and consequently require a source of osteocompetent cells. As a result, techniques have been developed for alveolar reconstruction that involved the preparation of the recipient site so that the allogeneic block can be inlaid into the recipient bed.
Demineralized Bone Matrix (DBM)
By demineralizing the freeze-dried bone to create what is referred to as demineralized bone matrix (DBM) or demineralized freeze-dried bone allograft (DFDBA), the implant loses its mechanical strength but becomes osteoinductive.76–78 Repeated studies have shown that removal of the mineral component from the bone matrix exposes proteins, referred to as BMP, which has been shown to induce bone formation at heterotopic sites (Figure 9-6). Osteoinductive capabilities of DBM make it a valuable tool for the surgeon working in the oral cavity. This biomaterial alone can be used to fill defects, extraction sites, and, in certain instances, onlay alveolar ridges. An early problem with this material was that it was available only in a granular or powder form. This meant that some form of membrane was necessary to retain the material, which increased costs and made the procedures more technically difficult.
Recent advances have seen DBM incorporated into various carriers such as collagen or selected polymers.79–81 The resultant entities, being either sponge-like or gel/putty-like in consistency, are easier to apply and are well retained within the recipient tissue bed. Applications for these products include periodontal infrabony defects, extraction sites to retain ridge form, alveolar ridge reconstruction, bone reconstruction associated with dental implant placement, bone reconstruction associated with dental implant complications, cyst or bony defects of the jaws, and periapical surgeries.82–92 In cases in which larger volumes of bone are required, such as maxillary sinus augmentation prior to dental implant placement, DBM may be used as a bone expander.93–95 More precisely, instead of looking to the iliac crest for an autogeneic bone graft, the surgeon can now consider using intraoral sites such as the mandibular symphysis or external oblique ridge and combine the bone harvested from this site with DBM.96 The use of bone mills designed to properly grind and prepare this bone assists the surgeon to facilitate this approach to reconstruction.
Xenogeneic Bone
Xenogeneic bone is osseous tissue that is harvested from one species and transferred to a recipient site of a different species.97,98 The most common implant of this type in clinical practice for bone reconstruction in the oral cavity is bovine-derived bone.99,100 Although other sources are available, such as murine or porcine bone, the availability of cow bone and the vast experience with it make bovine bone the most commercially viable. Xenogeneic bone is exceptionally popular for use in implant reconstruction. These products were introduced for implant reconstruction in the 1990s after years of careful scientific evaluation to address issues that plagued their initial launch in the 1960s, specifically involving the development of methods to further deproteinate bone particles.101 This processing reduces the antigenicity, making these implants more tolerable to the host tissues.102 The result is that the structure of bone is left to conduct new bone formation while the organic component of bone is almost completely removed. This anorganic bone matrix then has the structure of bone, making it osteoconductive without the osteoinductive or osteoconductive abilities imparted by the organic elements.
Research has shown that xenogeneic bone, if properly prepared, is well tolerated by the tissues. Eventually it will be replaced by host tissue, which makes it useful for defect or extraction site filling in the alveolus prior to dental implant placement or prosthetic rehabilitation.103–110 For oral applications it generally comes in a granular or powder form, which is somewhat difficult to handle. That form may also require some form of retentive structure such as a membrane to hold the material in the desired location.111–114
One interesting xenogeneic transplant, Biocoral (Biocoral, Inc., Wilmington, DE),115–117 is derived directly from the exoskeletons of corals from the group Madrepora of the genus Acropora. These corals are harvested from the relatively unpolluted waters of the reefs of New Caledonia. This is significant because corals from contaminated waters can contain petrochemical impurities. Both solid blocks and particulate implants fashioned from this material are composed largely of calcium carbonate and are osteoconductive. They are simultaneously incorporated into the human bony skeleton and replaced by human bone. The enzyme carbonic anhydrase, liberat/>
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses