Cement‐Retained Implant Restorations: Problems and Solutions
Chandur P.K. Wadhwani
Loma Linda University School of Dentistry, Loma Linda, California, USA
Dental practitioners have a preference for the cement‐retained implant‐supported restoration,1–3 essentially because it is more like the traditional tooth‐borne crown and bridge restorations with which they are familiar. Developed primarily to overcome issues with esthetics and occlusion, it also has benefits related to cost, potential for a better passive fit when dealing with a multiple‐unit prosthesis,4 and reduced chair time. Although dentists reported a preference for using cemented implant restorations, patients when surveyed did not appear to have any definite preference.5
Along with the reported benefits of using cemented implant restorations, a number of concerns emerged, for example there has been no technique for predictable removal and salvage of the restoration to access the abutment screw.6,7 Then, as implant therapy grew and popularity of cemented prostheses increased, implant site health issues started to emerge. For example, the influence on the type of implant restoration used and the general health of the surrounding soft tissues has been studied. Weber et al.5 conducted a prospective multicenter study on 152 implants placed in the maxillary anterior region. They concluded the peri‐implant soft tissues responded more favorably to screw‐retained crowns than to cement‐retained crowns. A recent systematic review also concluded that cement‐retained reconstructions exhibited more technical and biological complications compared with screw‐retained reconstructions.8 Pauletto et al.9 reported on complications associated with excess cement around crowns on osseointegrated implants in 1999, a series of four case studies. Tomson et al.10 and others11–16 followed suit with more case reports on issues relating to excess cement and peri‐implant disease.
Wilson17 evaluated 42 implants in 39 patients that showed signs of inflammation (peri‐implant mucositis or peri‐implantitis) and reported a positive link between excess cement and peri‐implant disease. This endoscopic study found excess residual cement associated with 81% of diseased sites. While the removal of the cement resulted in healing in most instances, further intervention was required in almost 25% of the cases. Most alarmingly, the time for the disease to be discovered varied from 4 months to almost 9.5 years after the crown was cemented. In January 2013 the American Academy of Periodontics, under the direction of the task force on peri‐implantitis, developed a position paper elucidating risk factors. Residual excess cement was highlighted as an etiological factor.18
Cemented restorations and issues related specifically to dental implants
The dental community has been using cemented restorations on natural teeth for well over 100 years19 with minor issues, provided the pulp and periodontal status are healthy. It is often questioned “why should it be when we use the same cement materials, with the same type of crown, place it with the same techniques for the implant restoration, adverse effects are reported which could potentially lead to the implant being lost?”
Tooth versus implants: biology
Implants have a very different mechanism of attachment to both the bone and the surrounding soft tissues compared with the natural tooth.20 A tooth crevice has keratinized epithelium at the base of the gingival sulcus, whereas an implant does not. The junctional epithelium is adherent and less permeable and has a high capability to regenerate around a tooth. Apical to the junctional epithelium lies the soft connective tissue attachment. The periodontium of a healthy tooth has a major advantage over the peri‐implant tissues in that the supracrestal collagen fiber bundles exist. Some of these insert directly into living cementum on the root surface of the tooth (Figure 12.1). This robust tooth–tissue interface has evolved over millions of years and serves as a seal to protect this area; it is highly protective against the ingress of bacteria and insult from trauma. Also, the arrangement of the periodontal fiber bundles results in a compartment‐like structure around the tooth which limits the spread of periodontal disease. By comparison, there is no cementum associated with the dental implant, so no direct collagen fiber insertion exists. Circumferential fibers that encircle the dental implant are present, behaving somewhat as a scar “cuff” that maintains the tissues in close apposition to the implant (Figure 12.2). This arrangement provides only one compartment that exists 360° around the implant. The single compartment around an implant explains why the associated inflammatory responses are generalized (Figure 12.3) rather than localized, as in the case of a tooth. The implant site that develops through healing within a few days is far more fragile, because the implant to soft tissue attachment mechanism is more of a cellular adhesion, being hemidesmosomal in nature.
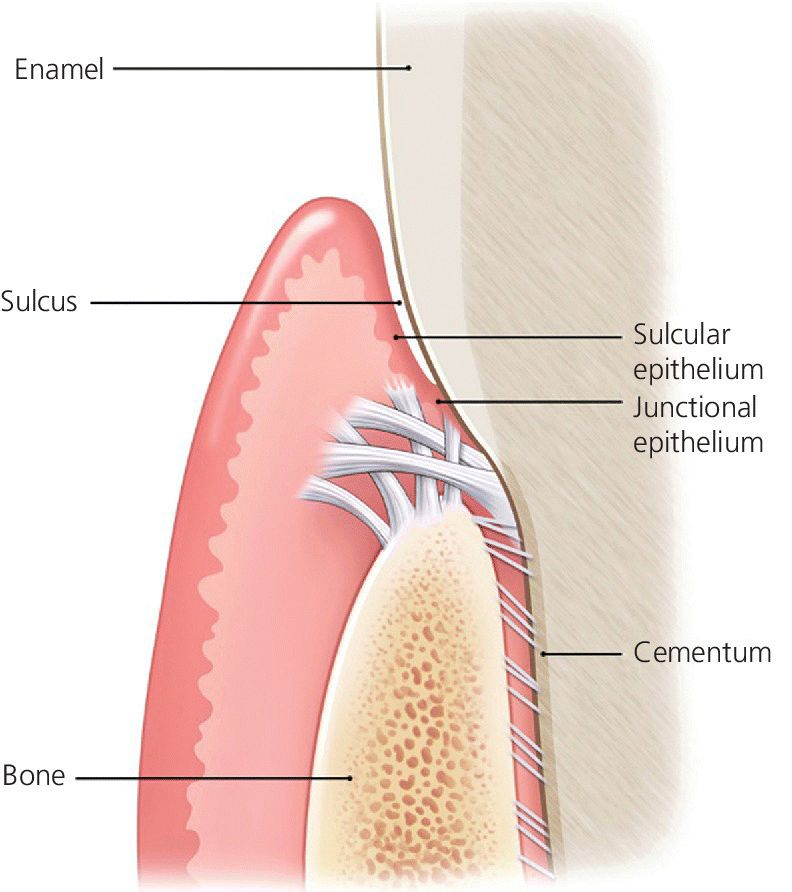
Figure 12.1 Biological attachment of a natural tooth. Connective tissue fibers insert into the root apical to the junctional epithelium.
Source: © 2016, Chris Gralapp
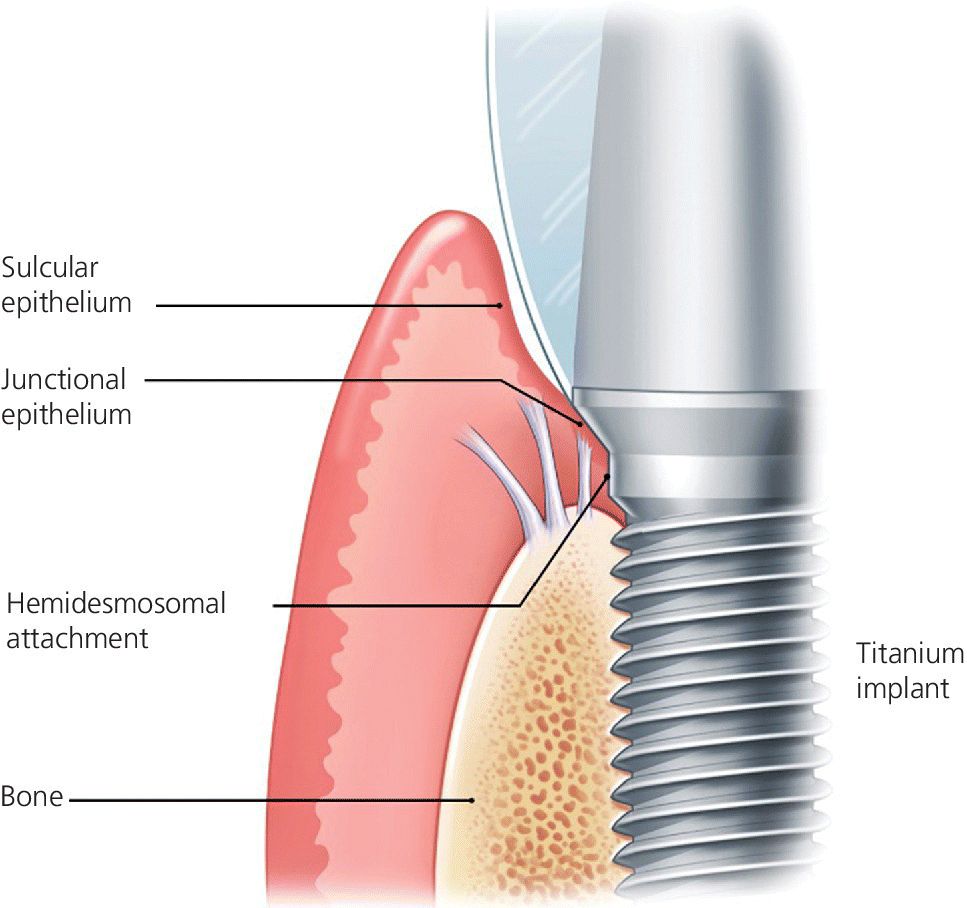
Figure 12.2 Biological attachment to a dental implant. There is no insertion of connective tissue fibers into the implant surface.
Source: © 2016, Chris Gralapp
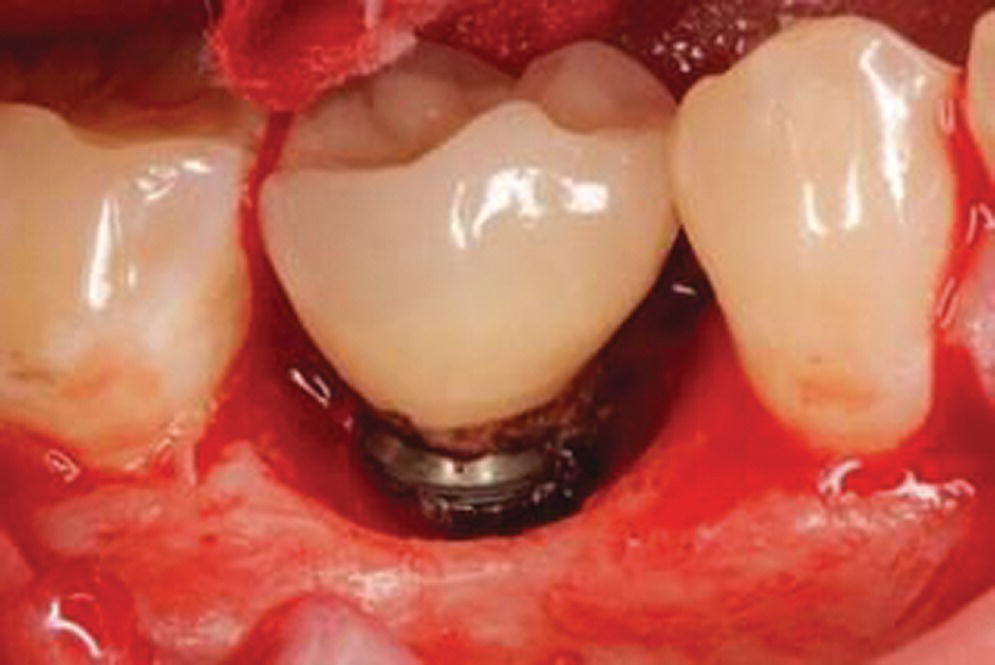
Figure 12.3 Example of Implant site with residual excess cement and associated peri‐implantitis. Note the generalized “crater” pattern of bone loss relates to the character of the biological attachment.
Source: reproduced with permission from Ken Akimoto, periodontist.
As a consequence, the connective tissue adhesion at implants is considered as having a poor mechanical resistance,21 with some authors considering that the mucosa at implants can hardly be qualified as “attached”.22
Implant depth and cement margin depth
In health, the implant–soft tissue attachment tends to occur at deeper levels when compared with the soft tissue attachment in the natural dentition. The platform of an implant, which is commonly flat topped, is frequently related to the facial crest of the soft tissues and is intentionally placed 3 mm deep to facilitate an emergence profile for the abutment and restoration. Where papillae exist, the flat top of the implant may now be located 5–7 mm deep to the crest of soft tissues.23 Maintaining and cleaning deeper than 4 mm is impossible with routine scaling instruments,24 and the microbiota at deeper sites tends to favor Gram‐negative bacteria, the type that are known to cause peri‐implant diseases.25,26 The depth of the implant site and associated bacterial challenge might contribute to a predisposition for a disease process, especially with residual excess cement.
When preparing the natural tooth for a cement‐retained restoration the cement finish line is rarely placed deeper than 0.5 mm into a healthy gingival sulcus, which is still considered a compromise.27,28 The prepared margins follow the outline of the gingival crest, moving more coronally to follow the outline of the papillae and remaining supragingival tissue wherever possible.29,30
Cement extrusion from the margin site during seating an implant crown can present problems with the ability to detect and remove this foreign material. Although anecdotal recommendations of 1–3 mm31,32 have been made regarding the acceptable depth a cemented margin may be placed relative to the tissue level, this has only recently been evaluated. The influence of the cementation margin position with respect to the amount of undetected cement was reported by Linkevicius et al.33 In a prospective clinical study they compared cement margin sites that were: equal with tissue level, 1 mm subgingival, 2 mm subgingival, and 3 mm subgingival. Although remnants were found at all depths, the cement average on the implant abutment and crown was ten times more when comparing equigingival to 1 mm subgingival. Statistically significant differences existed among the four groups: with the deeper the position of the margin, the greater the amount of undetected cement was discovered. The conclusions of the study indicated that, ideally, cement margins on implant abutments should always be visible for intraoral cementation.
The disease processes associated with residual excess cement are not understood. For example, it has not been determined if a threshold volume of cement extrusion is required, the location the cement reaches, or if the flow characteristics of the cement are individual or combined factors. The cement itself may also play an etiological role with microbial interactions, foreign body reactions, corrosion, and allergy (Figures 12.4 and 12.5).
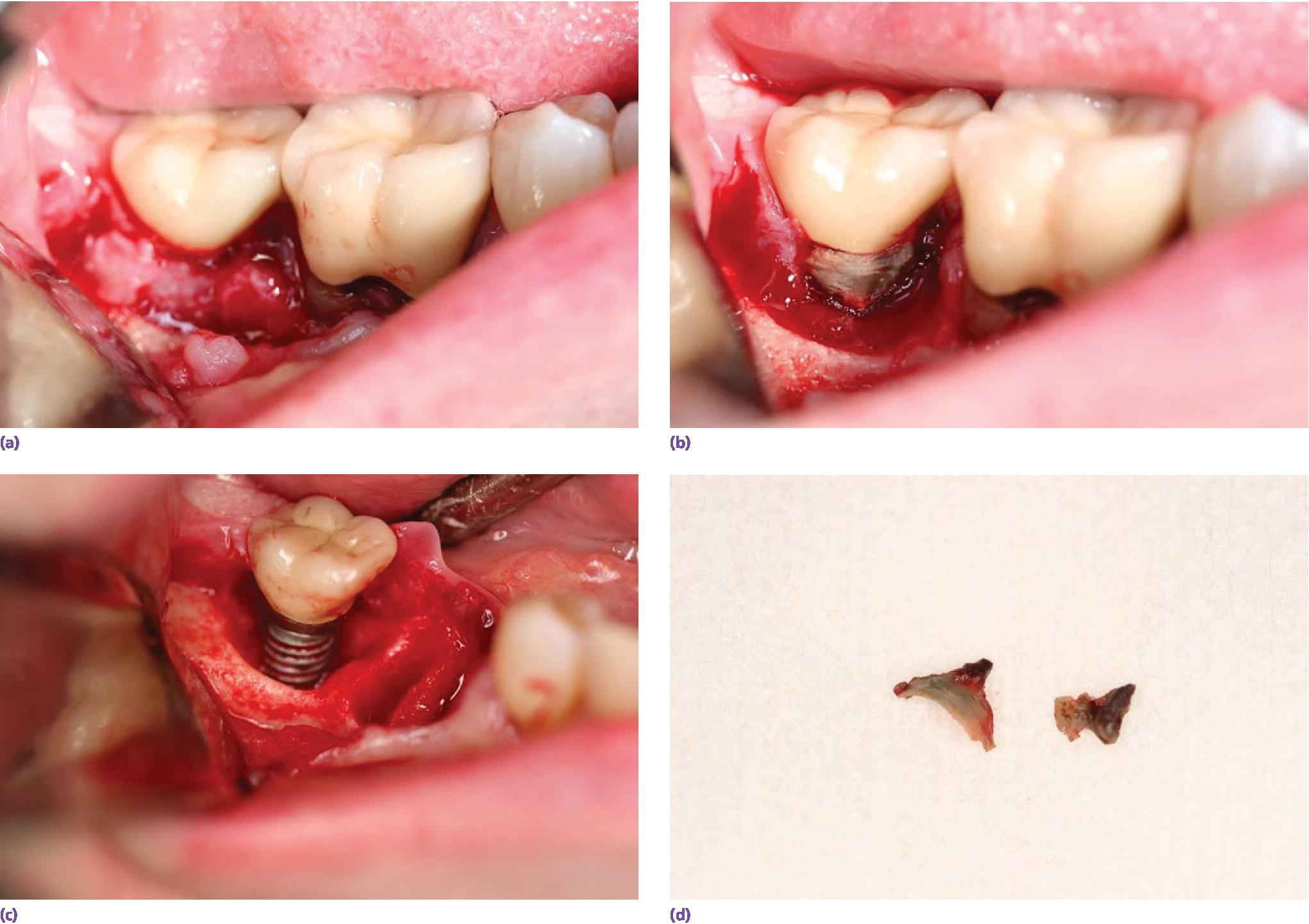
Figure 12.4 Series of photographs indicating the bone loss associated with an implant and residual excess cement and deep soft tissue cement margin. (a) Presenting with inflammation of the soft tissues. (b) Full‐thickness flap elevation – granulation tissue and cement evident. (c) Cement removed from implant and tissues. (d) Cement remnants.
Source: reproduced with permission from Franc Audia, OMS.
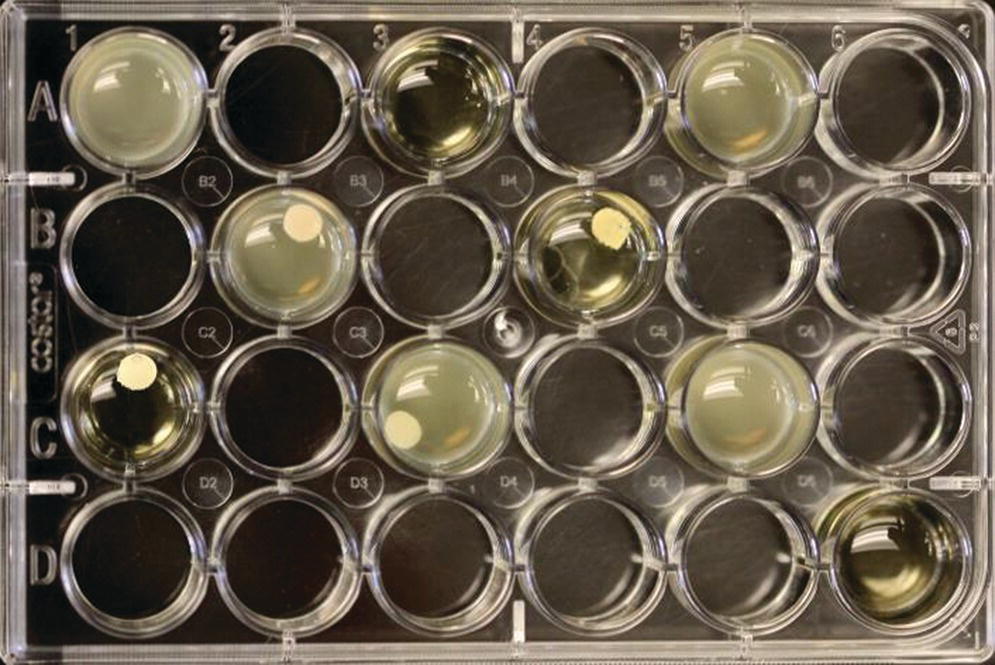
Figure 12.5 Example of test wells following 2 days of anaerobic incubation. F. nucleatum with four test cements, positive and negative controls are included in test. Note control wells – they contain no cement. The clear wells are the negative control (sterile).
Source: Ravel et al., 2015.40 Reproduced with permission of John Wiley & Sons.
Cement interactions: specific issues with implants
Microbial growth
The cementation of dental restorations is considered by many to be a simple unskilled task requiring little if any thought; in fact, the process is often delegated. Dental cements have been exclusively developed for teeth and as such have attributes such as antimicrobial agents, fluoride release, and adhesion properties all aimed at the natural tooth structures, where caries and bonding to dentin are demanding issues.34 Implants do not suffer from the effects of caries, nor do they have a structure based in dentin or enamel, so many of the properties desirable for the natural tooth are superfluous when considering dental implant restorations; in fact many of the properties optimized for a cement used for a natural tooth can be detrimental to an implant.35,36
Many studies have been undertaken to evaluate the antimicrobial effects of cements, however, they have been directed exclusively at how the materials behave in the presence of Streptococcus mutans and Lactobacillus acidophilius, which are the cariogenic microbes.34,37–39 There is little, if any, relevance for the implant restoration, where the effects of microbes that may result in peri‐implant disease are completely different and these should be the prime focus.
Raval and Wadhwani et al.40 designed a study with the periodontal department of the University of Washington to determine the interaction of implant luting cements and oral bacteria linked to peri‐implant disease. Specific luting cements were exposed to peri‐implant disease‐producing microbes. The five luting cements that were tested were as follows:
- zinc oxide–eugenol (TempBond Original, TB; Kerr, Orange, CA, USA);
- eugenol‐free zinc oxide (TempBond Non‐ Eugenol, TBNE; Kerr);
- zinc orthophosphate (Fleck’s, FL; Keystone Industries, Cherry Hill, NJ, USA);
- methacrylate‐based resin (Premier Implant Cement [PIC; Premier, Plymouth Meeting, PA, USA] and Multilink Implant [ML; Ivoclar Vivadent Inc., Amherst, NY]).
Disks 5 mm in diameter were made from each cement, under carefully controlled conditions to prevent contamination. The disks were made with similar surface configurations to be macroscopically smooth. A planktonic growth evaluation used bacterial species of Aggregatibacter actinomycetemcomitans, Fusobacterium nucleatum, and Porphyromonas gingivalis, grown individually in modified TYK broth (trypticase soy broth, yeast extract, vitamin K) in test wells. The concentration of bacteria was standardized for each test with measurements made using an optical spectrometer, which related the opacity of the broth, such that the greater the broth opacity the greater the microbial content. Statistically significant differences were found among the cement groups, with TempBond Original consistently providing results no different to the media alone at the P < .05 significance level. The results for A. actinomycetemcomitans (Aa), F. nucleatum (Fn), and P. gingivalis (Pg) are consistent in this respect (Figure 12.6).
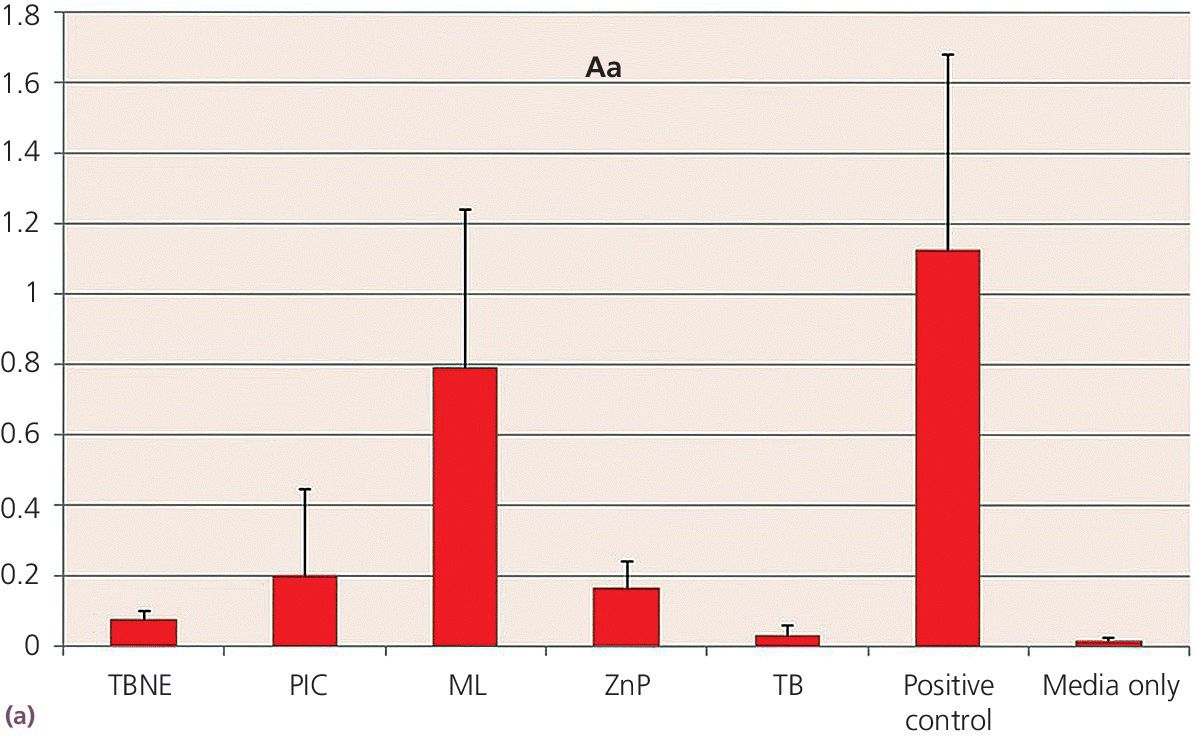
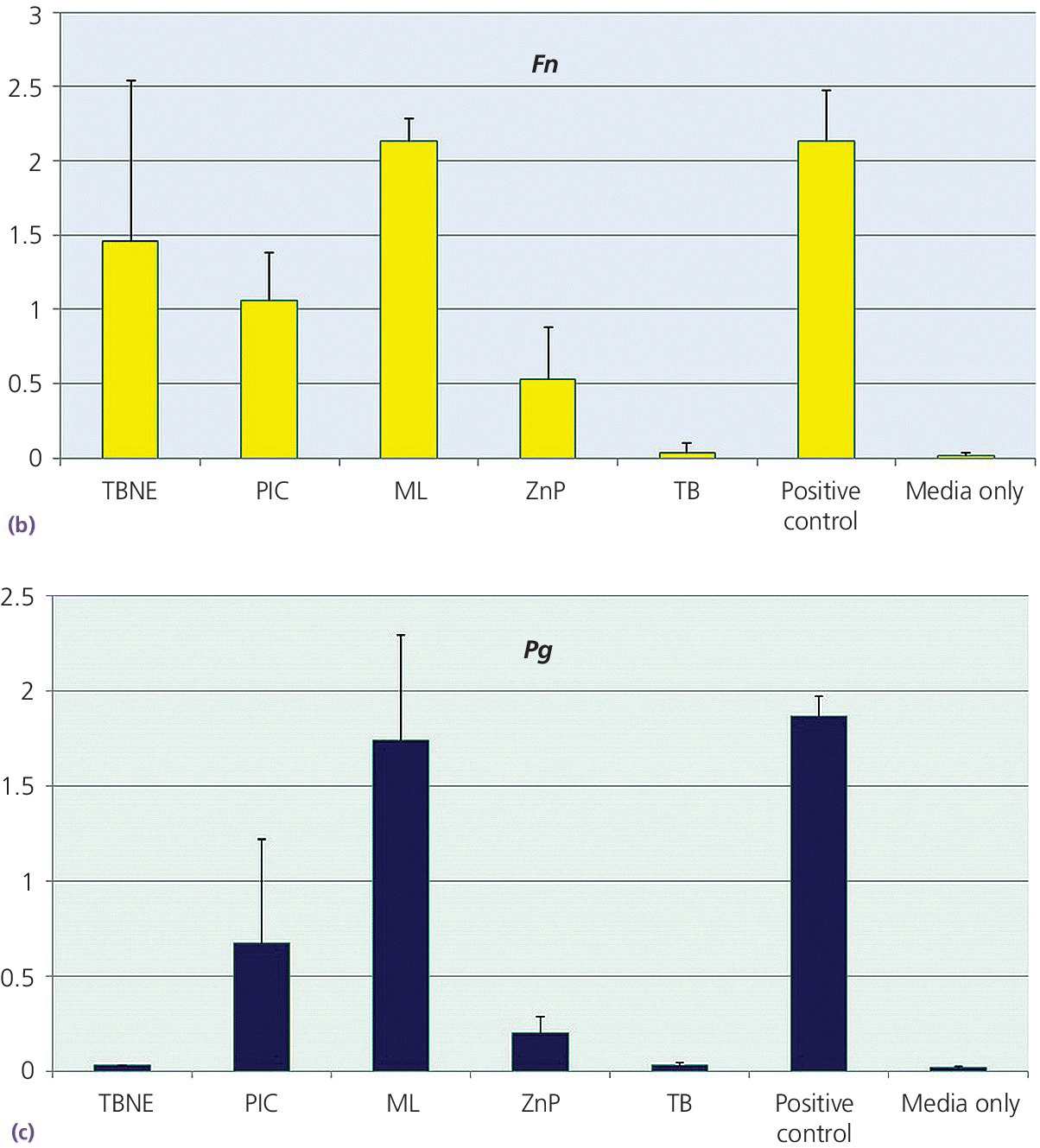
Figure 12.6 Planktonic growth measurement on different cements by opacity test (OD 600) values. (a) Aggregatibacter actinomycetemcomitans – Aa. (b) Fusobacterium nucleatum – Fn. (c) Porphyromonas gingivalis – Pg. FL, Fleck’s Cement; ML, Multilink Implant; PIC, Premier Implant Cement; TBNE, Temp‐Bond Non‐eugenol; TBO, TempBond Original. Positive control (media with bacteria, no cement) and negative control (sterile media) also shown.
A second part of this study involved how these anaerobic Gram‐negative microbes grew as a biofilm on the test cement disks. Here the cement disks were removed from the TYK and bacterial solutions, washed with a sterile solution of TYK, and also agitated to remove the more adhered bacteria. The resulting solution was used to coat agar plates then incubated for 4 days anaerobically. Colony‐forming units (CFUs) were recorded for each bacterium–cement combination. This experiment was repeated three times. The biofilm test was used to compare the cement that inhibited the planktonic growth the most (TB) to the one that inhibited the least (ML). Again contrasting results were obtained, with TempBond Original consistently giving no CFU counts, and Multi‐Link implant cement giving CFUs too numerous to count (these were greater than 5000 in many instances) (Figure 12.7).
Source: Raval et al., 2015.40 Reproduced with permission from John Wiley & Sons.
This simple but enlightening study indicated that cement selection should be considered with respect to how it may impact the environment in which it is being placed. This is particularly relevant for a patient who is periodontally susceptible, as these patients present a greater risk of peri‐implant disease.18 Consistent with this is the observation that in most peri‐implantitis cases, the composition of the flora is similar to that of the subgingival flora of chronic periodontitis.25,26
It has also been demonstrated that the bacteria in cases of partially edentulous implants may be more pathogenic (especially Gram‐negative rods and spirochetes) than in the fully edentulous case, indicating a possible seeding mechanism from tooth pocket to implant crevice.28 The cements that performed the best overall within this study had the common element of zinc. Zinc is known to be highly effective as an antimicrobial and eugenol is also well known for its anti‐inflammatory, anti‐oxidative, and antimicrobial pharmaceutical properties; the combination of these two components is likely the reason for the effectiveness of TempBond Original.
Host response: foreign body reaction
Histopathological evaluation of the soft tissues associated with failed implants has found cement particles within the soft tissue specimens.41,42 Residual cement may act as a nidus for both acute and chronic inflammatory cells, which in turn may elicit a cascading autoimmune destructive process. Further studies have identified the types of cement that have been found within the tissues using scanning electron microscopy coupled to energy dispersive X‐ray spectral analyzers, capable of determining the composition of the foreign material encountered.43 Using commercially available cements as a standard, the particles in each biopsy specimen could be associated with one of the commercial cements with a level of probability ranging between 0.79–1. Table 12.1 shows the results of 19 soft tissue biopsy specimens related to implant failure where residual excess cement particles were detected; the cements were identified and quantified.
Table 12.1 Cement types identified in 19 soft tissue biopsy samples of failed, removed implants.
| Cement brand and nomenclature | Manufacturer | Number of cases (19 biopsy specimens) |
| Telio CS Cem Implant (Telio) | Ivoclar Vivadent (Amherst, NY) | 7 |
| TempBond clear with triclosan (TB) | Kerr Sybron Dental Specialties (Orange, CA) | 1 |
| Premier® Implant Cement (PIC) | Premier (Plymouth Meeting, PA) | 4 |
| RelyX™ Unicem 2 Self‐Adhesive Resin Cement (Relyx1) | 3 M ESPE (St. Paul, MN) | 4 |
| Intermediate Restorative Material (IRM) | Dentsply (Waltham, MA) | 3 |
The study concluded that the particles found in human soft tissue biopsy specimens around implants affected by peri‐implant disease occurred with a variety of cements. No data quantified the individual amounts of cement found within the tissues, which could be a confounding factor.
The question how the cement particles became embedded within the tissues is speculative. It may have resulted from the time the crown was initially seated: with sufficient force the mass of liquid cement could have disrupted the fragile hemidesmosomal attachment of the epithelium to the implant. It may have resulted during clean up, with the dislodgement of the set particles being forced into the tissues.
Host response: allergic response
Dental cement manufacturers continue to develop cements based to a large degree on their retentive capabilities. As noted previously, cements have evolved from use with natural teeth, where the primary elements involved are dentin and enamel. Resin‐modified glass ionomers (RGMI) have become popular due to their ability to adhere chemically to dentin and the potential for fluoride release. In 2010 an estimated 73% of dental schools also used this material as the definitive medium for cementing restorations to implants,44 which interestingly followed a similar pattern for cement selection for teeth (Figure 12.8). One of the constituents of the RMGIs is hydroxyethyl methacrylate (HEMA).45 This material is extremely irritating to the tissues to the extent that the material safety data sheet (MSDS) advises that gloves and other mucosal tissue, such as eyes, be barrier protected when it is used. With the natural tooth, where preparation finish lines are supragingival and isolation with rubber dam is feasible, this presents no real issue. However, with implants, barrier protection is more difficult to achieve, the epithelial attachment adheres poorly, is more permeable, and may be more susceptible to the effects of chemicals leaching out from materials.
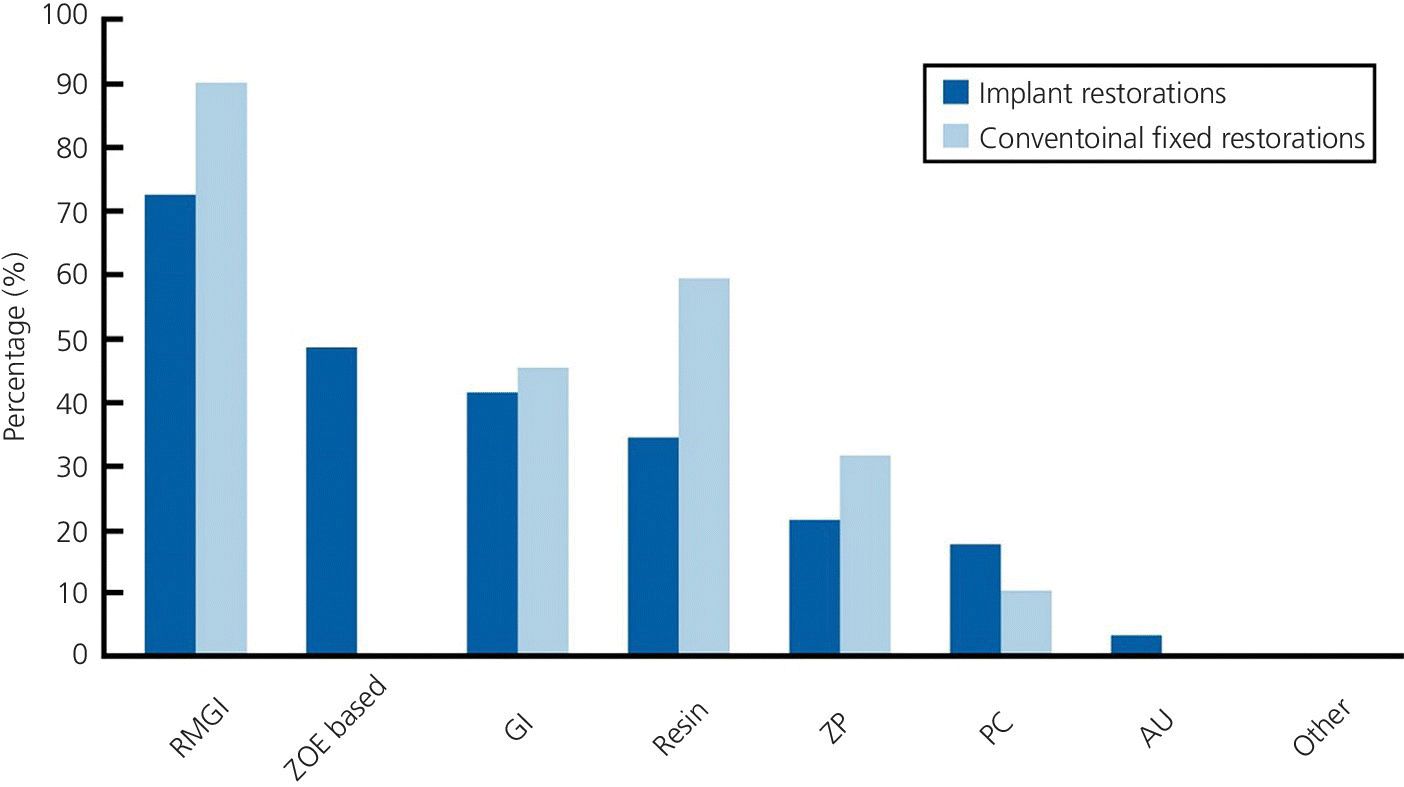
Figure 12.8 Data graph on definitive cements used for implant and tooth restorations by USA dental schools. (RMGI, resin modified glass ionomer; ZOE, zinc oxide eugenol; GI, glass ionomer; ZP, zinc phosphate; PC, polycarboxylate; AU, acrylic urethane).
Source: Tarica et al., 2010.44 Reproduced with permission from Elsevier.
Corrosion
Much of the success of dental implants relates to the relatively low corrosive susceptibility of the materials involved. Titanium appears well suited to the oral environment under most conditions.
However, recent reports have found a reactive issue with some materials commonly found in dental cements.35,46,47 Fluoride, the ubiquitous anti‐caries agent, is known, in some forms, to corrode titanium.48 One study found that several cements containing the fluoride ion, under acidic conditions, caused pitting corrosion when in contact with titanium (Figure 12.9).49 Corrosive elements are generally undesirable as the surface alterations produce an increased adhesion to peri‐implant disease‐producing anaerobic bacteria,50 and also result in reactive oxidative species being produced by the host.51 The inflammatory response produced may be one mechanism that results in peri‐implant disease. The cements identified as producing the conditions for corrosion include some polycarboxylates and some glass ionomer cements.49,52 Durelon (3M‐ESPE) alludes to this problem in the Instructions For Use (IFU),49 which is of interest as 17% of US dental schools44 admit to using it on implant restorations. This indicates the IFU are not being followed or may not even be read.
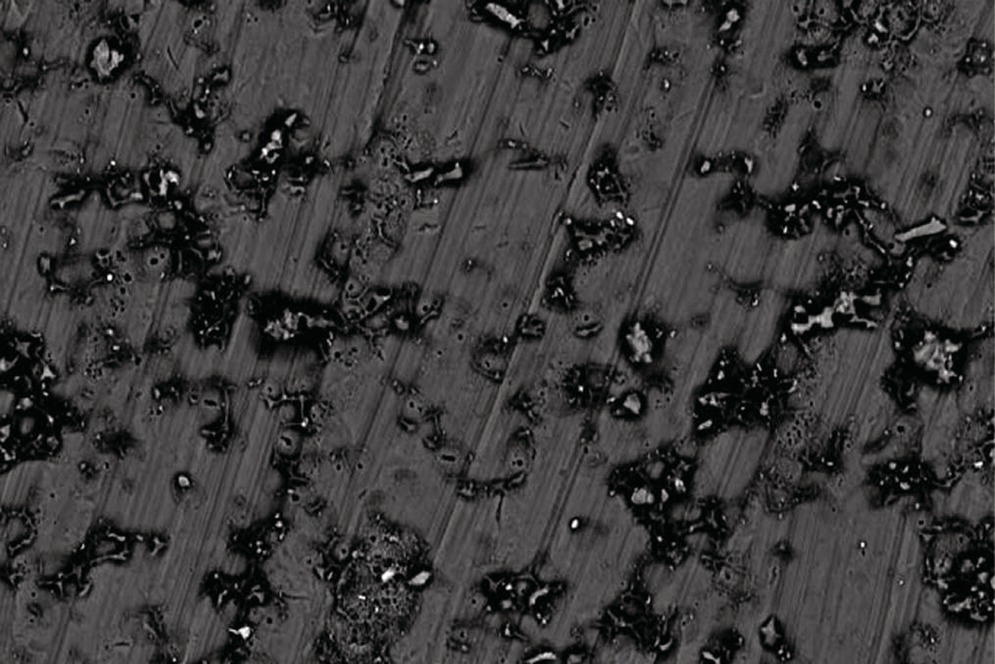
Figure 12.9 Scanning electron microscope image of the surface of a turned titanium alloy disk – the machined marks are visible as concentric lines. The surface shows pitting corrosion after the application of Durelon cement. This surface pitting perpetuates the corrosion over the electrochemically active heterogeneous surface.
To date, no ideal implant luting cement exists, all have the potential to cause issues. However, the prudent clinician will evaluate carefully the material used, will elevate the cement margin to be readily accessible to cleaning, and have a good biological understanding of the limitations of the dental implant.
Luting material selection
Desirable properties for implant cements include ease of clean up and removal of excess cement; studies have indicated this to be especially challenging with resin cements.53 The greater the retentive properties a cement has for titanium the more difficult it will be to remove. Also, the greater the mechanical retention of a given cement to the implant materials used, the more difficult it will be to clean excess cement from them. Table 12.2 outlines some of the more appropriate properties required of implant cements and compares these to cement properties more ideal for natural teeth. Desirable properties such as the noncorrosive nature and antimicrobial properties have been discussed. Excellent radiographic density is also a valuable asset for an implant cement (Figure 12.10).13,54
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


