Biochemistry, Nutrition, and Nutritional Counseling
This chapter reviews the six major nutrient groups and their metabolic activities in mammalian cells, dietary modifications for diseases, nutritional diseases and disorders, and oral manifestations of nutritional deficiencies and toxicities. The effects of nutrients on oral tissues and the dietary assessment tools and techniques available for counseling individuals with various types of oral diseases are also described. Because nutritional problems in the developed countries are a result of overeating and undereating, a review of energy balance and weight control is included. Cellular biochemistry is fundamental to the study of nutrition; therefore, the reader is referred to the “General Histology” section in Chapter 2 for a review of structural and functional similarities in cells.
Six Major Classes of Essential Nutrients
Carbohydrates
A Definition—polyhydroxy aldehydes or ketones that serve as the body’s primary sources of quick energy; carbohydrates (CHO) are composed of monosaccharides, basic units that contain carbon, hydrogen, and oxygen
1. The ratio of carbon, hydrogen, and oxygen is 1 : 2:1
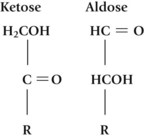
2. The reactive portion of the molecule may be in a ketose form or an aldose form
3. The position of the hydroxyl (–OH) groups determines properties such as sweetness and absorbability
(1) Trioses (C3) and tetroses (C4)—usually formed during intermediary metabolism and are not important dietary components
(2) Pentoses (C5)—important in nucleic acids and coenzymes; do not occur in free form (uncombined); not important dietary components (e.g., ribose)
b. Disaccharides—composed of two monosaccharide units
(1) Sucrose—glucose plus fructose (e.g., cane and beet sugar)
(2) Lactose—glucose plus galactose (e.g., milk sugar)
(3) Maltose—glucose plus glucose (intermediate of starch hydrolysis [digestion])
c. Oligosaccharides—composed of two to six monosaccharide units
a. Homopolysaccharides—made up of more than six identical monosaccharide units
(1) Starch—plant storage form of glucose; source of half of dietary carbohydrates
(2) Glycogen—animal storage form of glucose; found in the liver and muscle of living animals; insignificant source of dietary carbohydrates
(3) Cellulose—chief constituent of the framework of plants; glucose units are in β-linkages, not capable of being hydrolyzed by human digestive enzymes; provides bulk and fiber in the diet
b. Heteropolysaccharides—carbohydrates associated with noncarbohydrates or carbohydrate derivatives
(1) Pectin, lignin—important contributors to fiber in the diet
(2) Glycoproteins—carbohydrate and protein in a specific, functional arrangement (e.g., blood group substances and many hormones)
(3) Glycolipids—carbohydrate and lipid, as in gangliosides
(4) Mucopolysaccharides—protein and carbohydrate in a loose binding
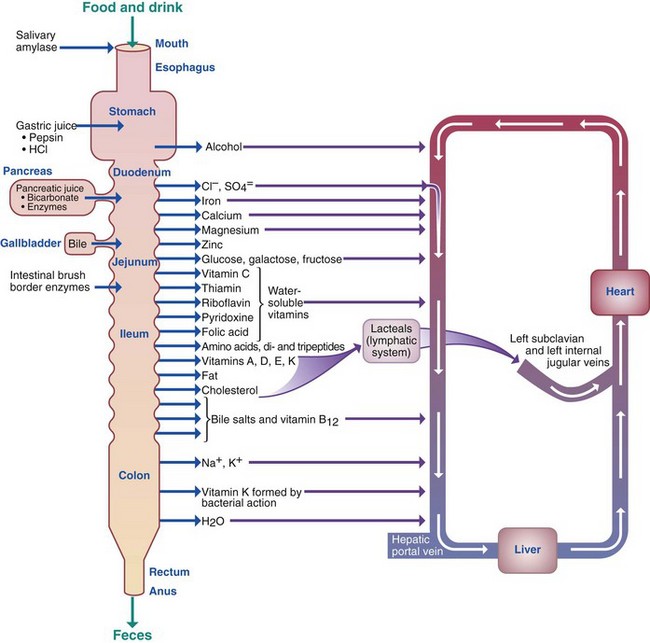
D Digestion, absorption, and transport
TABLE 12-1
Digestive Action at Various Points Along the Gastrointestinal Tract
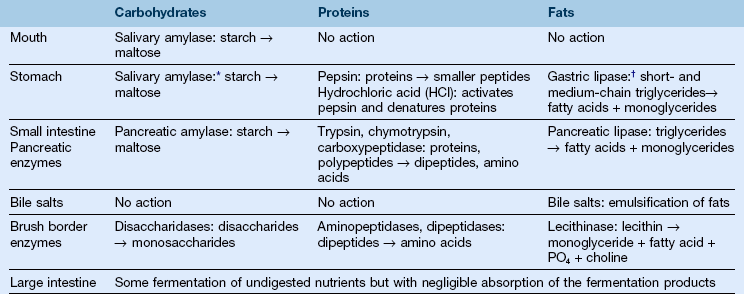
*A small amount of action within the bolus of swallowed food.
(1) Teeth and tongue—mechanical breakdown and mixing of food
(2) Saliva—hydration and lubrication of food
(3) Salivary amylase (ptyalin)—initial enzymatic hydrolysis of starch
b. Stomach—no digestive enzymes for carbohydrates; initial enzymatic hydrolysis of starch by salivary amylase may continue
d. Large intestine—bacterial “fermentation” of some undigested carbohydrates
a. Factors affecting absorption
(1) Passive diffusion along the osmotic gradient—when the intestinal concentration of carbohydrates is greater than the level of carbohydrate in the blood
(2) Facilitated diffusion—only certain molecules allowed to pass across a membrane using an ion channel or a carrier protein
(3) Active transport—requires energy and allows molecules to pass against a concentration gradient with the aid of an ion channel or a carrier protein at the brush border
E Metabolism—glucose is the main immediate source of energy for the body; a glucose level of 70 to 120 mg/100 mL blood is maintained by most healthy persons (Figure 12-2)
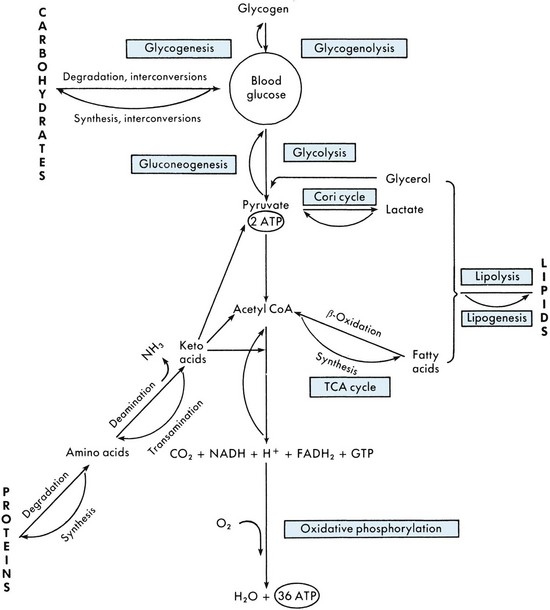
FIGURE 12-2 An overview of metabolism.
a. Dietary carbohydrates—sugars, starches
b. Stored liver glycogen breakdown—glycogenolysis
c. Synthesis from intermediary metabolites such as pyruvic acid—glyconeogenesis
2. Reactions of blood glucose—”burned” (oxidized) for energy
a. Glycolysis—end product is pyruvate or lactic acid in the absence of oxygen (anaerobic conditions) or acetyl–coenzyme A (acetyl-CoA) in the presence of oxygen (aerobic conditions)
b. Tricarboxylic acid cycle (TCA) or Krebs cycle—oxidation of acetyl-CoA with the release of carbon dioxide (CO2)
c. Oxidative phosphorylation and electron transport—production of adenosine triphosphate (ATP, a high-energy molecule) and water
1. Anabolic hormones—lower the blood glucose level (e.g., insulin)
2. Catabolic hormones—raise the blood glucose level
a. Glucagon—stimulates glycogenolysis
b. Steroid hormones—stimulate gluconeogenesis
c. Epinephrine—stimulates glycogenolysis
d. Growth hormone and adrenocorticotropic hormone (ACTH)—act as insulin antagonists
e. Thyroxine—increases insulin breakdown, intestinal absorption of glucose, and epinephrine release
3. Coenzymes—B-complex vitamins are important precursors of the coenzymes involved in the catabolism of carbohydrates
1. Definition—substance, usually nonstarch polysaccharide, found in plants; not broken down by human digestive enzymes; some of it is digested by bacteria in the gastrointestinal (GI) tract
a. Insoluble fiber—substance (e.g., cellulose, hemi-cellulose, and lignin) that gives structure to plant cell walls; adds bulk and softness to stools; reduces contact with possible carcinogens by decreasing transit time through the colon; foods high in insoluble fiber include wheat bran, raw fruits, and vegetables
b. Soluble fibers—substances (e.g., gums, mucilages, pectin, and oat bran) that dissolve to become gummy or viscous; lower blood cholesterol; regulate the use of sugars and slow down gastric emptying; foods high in soluble fiber include legumes, raw apples, and whole oats
2. Epidemiologic studies indicate that individuals whose diets include a significant amount of fiber have a low incidence of chronic “Western” diseases, for example, coronary heart disease, diabetes, atherosclerosis
3. Specific fibers are believed to play roles in decreasing the incidence of obesity, irregularity, hemorrhoids, appendicitis, diverticulosis, colon cancer, hyperlipidemia, and fluctuations in blood glucose (Table 12-2)
TABLE 12-2
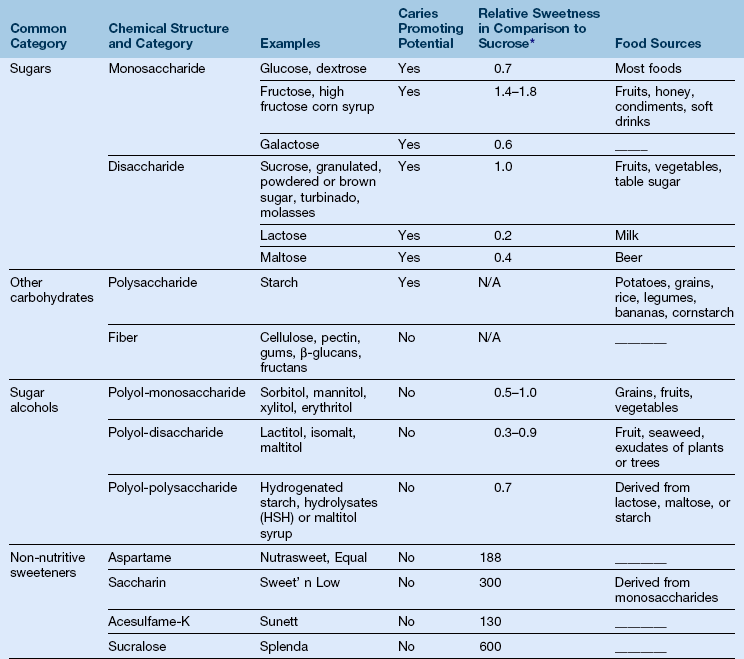
Adapted from Hubrich B, Nabors LO. Glycemic Response. In Formulating Glycemic Strategies, a supplement to Food Product Design. July 2006, pp 3–17. July 2006, pp. 3–17.
a. For persons with a limited intake, diets high in fiber bulk may cause nutritional deficiency
b. Use of large doses of purified fiber may inhibit absorption of calcium, potassium, zinc, and iron
c. Phytic acid, often found in high-fiber foods such as cereal grains, can bind and prevent absorption of minerals such as iron, calcium and zinc
5. Recommended fiber intake—for adults, 20 to 30 grams per day (g/day); an upper limit of 35 to 40 g/day is recommended for individuals with a family history of diet-implicated cancer; a limit of 50 g/day is recommended for diabetics
H Biologic role and functions of carbohydrates
1. Provide precursors of structural and functional molecules (e.g., gangliosides)
1. Pre-eruptive effect on teeth
2. Post-eruptive effect on teeth
a. Energy source for oral cariogenic bacteria (e.g., Streptococcus mutans)
b. Acidogenic bacteria metabolize monosaccharides and disaccharides, particularly sucrose, for the production of energy through glycolysis that results in the formation of lactic acid, pyruvate, and other acetyl–CoA–dependent on the conditions
c. S. mutans synthesizes polysaccharides (glucans, levans, and glycogen) from sucrose
(1) Polysaccharides are used for energy when sucrose is unavailable
(2) Glucans form insoluble complexes with S. mutans and have a strong affinity for enamel, thus enhancing bacterial plaque formation
(3) The organic acids are liberated into the interface between the bacterial plaque and surface enamel
d. The firm texture of some complex carbohydrates, as found in raw fruits and vegetables, can help to remove food debris retained between teeth; the chewing action can also stimulate salivary flow
3. Dietary sweeteners (see Table 12-2)
a. Nutritive sweeteners are used by the body as an energy source; they provide calories
(1) Sugar alcohols (xylitol, sorbitol, and mannitol) are noncariogenic nutritive sweeteners that are slowly fermented through anaerobic metabolism by oral bacteria; excessive intake of these polyols can cause diarrhea because of the osmotic transfer of water into the bowel
(2) Xylitol is found naturally in plants and is equal to or sweeter than sucrose. Consumption of xylitol-containing products following consumption of food has been shown to interfere with the metabolism of S. mutans and decrease the demineralization of enamel
b. Nonnutritive sweeteners are calorie free and have no nutritive value; aspartame, saccharin, and acesulfame-K are nonnutritive sweeteners approved by the U.S. Food and Drug Administration (FDA); are noncariogenic
c. Aspartame should be avoided by patients who have phetylketonuria, a genetic disorder characterized by an inability to metabolize the amino acid phenylalanine
d. Food labels often list sugar content in its various forms (e.g., invert sugars, dextrose, fructose, corn sweeteners) to give an appearance of lower sugar content
4. Cariogenicity factors of diet habits (from most to least important)
a. Intake frequency of simple sugars—the more frequent the exposure to sugar, the more cariogenic is the diet; six candy bars eaten at six different times during the day are more harmful in terms of acid and bacterial plaque formation than six candy bars consumed at the same time
b. Form of simple sugars (liquid or retentive)—liquid sweets clear the oral cavity faster than solid or retentive sweets do and therefore are less cariogenic
c. Time of ingestion of simple sugars—combining sweets with liquids and other noncariogenic foods during a meal is less cariogenic than a concentrated exposure to sweets between meals as a snack
d. Total intake of simple sugars—average daily intake of sugar is 22 teaspoons; the majority of our simple sugar intake comes from soft drinks, fruit drinks, desserts, candies, and ready-to-eat cereals. The American Heart Association (AHA) recommends 6 teaspoons per day for women and 9 teaspoons for men1
e. Starch-rich foods that are retained on the teeth for prolonged periods are ultimately degraded to organic acids and can contribute to the production of dental caries
f. Combining cariogenic foods with noncariogenic foods—recent studies indicate that certain cariogenic foods (e.g., canned pears in syrup) are less cariogenic when combined with a particular noncariogenic food (e.g., cheese)
5. Importance of carbohydrates in periodontal health
a. Energy source for the growth and repair of periodontal tissues
c. Firm texture of complex carbohydrates can promote circulation in gingival tissue
d. Dietary monosaccharides and disaccharides enhance supragingival bacterial growth and plaque formation; these bacteria set the stage for the growth and development of subgingival bacteria and plaque, which are responsible for the destructive effects of periodontitis
1. 130 g/day of digestible carbohydrate is the recommended daily allowance (RDA) for adults and children. Minimum adult intake (50 to 100 g of digestible carbohydrate) prevents use of body protein as an energy source; pregnant and lactating women need additional carbohydrates to prevent ketosis
a. The Food and Nutrition Board recommends that 45% to 65% of calories should come from carbohydrates2
b. Calories from simple carbohydrates (monosaccharides and disaccharides)—10% or less of the total caloric intake
c. The majority of calories should come from complex carbohydrates (including fiber)
K Dietary modifications for persons with disease conditions
1. Obesity—reduce total calories and percentage of simple carbohydrates (concentrated sweets) to increase the nutrient density of a lower-calorie diet
a. Lactose intolerance (inability to hydrolyze lactose)—eat fewer milk products, use fermented products, or add a commercial lactose enzyme (lactase) to milk
(1) Yogurt with active-bacteria culture is recommended because the lactose is digested by the yogurt
(2) The main concern for oral and systemic health is an inadequate intake of calcium and vitamin D; a hydrogen breath test can be used for diagnosis
b. Galactosemia—congenital inability to metabolize galactose; lactose and milk products should be removed from the diet
c. Fructose intolerance—congenital inability to metabolize fructose; fructose and sucrose should be removed from the diet; individuals with fructose intolerance have significantly fewer dental caries
3. Dental caries and periodontal disease—a protective diet should be implemented
a. A diet that is low in retentive carbohydrates
b. Avoidance of cariogenic snacks
c. A diet that is adequate in all nutrients (Table 12-3)
TABLE 12-3
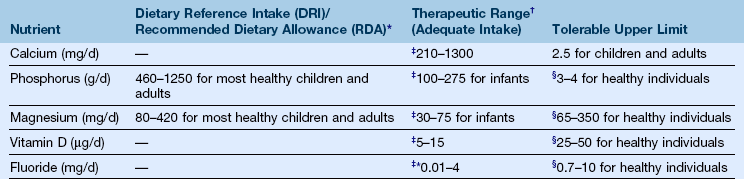
*Adequate intake (AI), also known as a therapeutic range, is the mean intake for healthy individuals that is used when an RDA value cannot be determined.
†Recommended dietary allowance values meet the needs of 97% of individuals in a group. Daily reference intake values are groups of values that provide quantitative estimates of nutrient intake for planning and assessing diets for all healthy individuals.
‡The lower number represents the adequate intake for infants and children, and the higher range of numbers will vary depending on life stage and gender group. Refer to the National Academy Press Web site (http://nap.edu/) for more in-depth information. The therapeutic range, also referred to as adequate intake, is the dose at which physiologic benefits for healthy individuals and decreased risk for toxicity may exist.
§The lower number represents the upper limits for infants and children, and the higher number represents upper limits for males and females (pregnant and lactating). The tolerable upper limit is the highest level of daily nutrient intake that is likely to pose no risk of adverse health effects.
4. Diabetes (inability to regulate glucose because of insufficiency or relative ineffectiveness of insulin)—dietary treatment (see the section on “Diabetes Mellitus” in Chapter 19)
b. Signs and symptoms of diabetes mellitus
(1) Frequent urination (polyuria)
(2) Excessive thirst (polydipsia)
(a) Is managed primarily with insulin therapy
(b) A regular pattern of three meals per day, with one or more snacks between meals
(c) A diet that is rich in complex carbohydrates and dietary fiber
(d) A diet high in carbohydrates replaced with unsaturated fat and dietary fiber, if elevated triglycerides are present
5. Reactive hypoglycemia—rare; symptoms of dizziness, hunger, and heart palpitations are lessened with a low-carbohydrate diet
6. Dumping syndrome—occurs after gastric surgery; postprandial symptoms of nausea, dizziness, cramping, and diarrhea are lessened by a low-monosaccharide, low-disaccharide diet
7. Alcoholism—overconsumption of alcohol may cause malnutrition (see the section on “Chronic Alcohol Abuse and Dependence” in Chapter 19)
b. Empty-calorie food—provides energy but few other nutrients (e.g., concentrated sweets, alcohol, and fats)
c. Causes vitamin B depletion because the liver needs niacin and thiamine to metabolize alcohol
d. Causes folate and iron deficiency
e. Depresses antidiuretic hormone, causing loss of magnesium, potassium, and zinc in urine
8. Alcohol consumption during pregnancy—has a direct teratogenic effect on the developing fetus: fetal alcohol syndrome (see the section on “Fetal Alcohol Spectrum Disorders” in Chapter 19)
9. Carbohydrate regulation in some hyperlipoproteinemias—total carbohydrate and alcohol intake is controlled; concentrated sweets are restricted
Proteins
A Definition—complex biologic compounds of high molecular weight that contain nitrogen, hydrogen, oxygen, carbon, and small amounts of sulfur; each protein has a specific size and is made up of amino acid building blocks linked through peptide bonds in a specific arrangement
a. Simple proteins—contain amino acids only
b. Compound (conjugated) proteins—contain simple proteins and a nonprotein group
c. Derived proteins—fragments produced during digestion or hydrolysis (e.g., peptides, peptones, and proteases)
a. Complete proteins contain sufficient amounts of the essential amino acids for normal metabolic reactions; found in foods of animal origin
(1) A total of 20 essential amino acids that cannot be synthesized by humans and must be provided in the diet in sufficient amounts to meet the body’s needs
(a) Adult—histidine, isoleucine, leucine, lysine, methionine, phenylalanine, threonine, tryptophan, and valine
(b) Infant—all of the above plus histidine and probably taurine
(2) Nonessential amino acids can be synthesized by the body and need not be provided by the diet but are necessary for normal metabolic reactions; include alanine, arginine, aparagine, aspartic acid, cysteine, glutamic acid, glutamine, glycine, proline, serine, and tyrosine
b. Incomplete proteins have insufficient quantities of one or more essential amino acids to support protein synthesis in humans; plant proteins are often incomplete (e.g., corn protein is low in lysine; legume protein is low in methionine)
c. Complementary proteins are proteins that are incomplete when ingested singly but, when combined, provide sufficient essential amino acids
(1) In a “vegan” (or strict vegetarian) diet, the complementing of plant proteins can be accomplished by combining appropriate incomplete proteins; the amino acids in different foods can complement one another, even when eaten at different meals; persons on a strict vegetarian diet are at the greatest risk for developing deficiencies in calcium, iron, zinc, and vitamin B12 because the major food sources of these nutrients come from animal products
(2) In an “ovo-lacto” vegetarian diet, milk and egg proteins can provide the essential amino acids that are inadequate in incomplete plant proteins; however, this diet still may be deficient in iron
d. Protein quality is a measure of a protein’s ability to support protein synthesis; it is measured by comparing the test protein with a reference protein, usually egg protein
(1) Amino acid or protein chemical score (CS)—compares the essential amino acid content in a dietary protein to that of a reference protein
(2) Protein efficiency ratio (PER)—measures a protein’s ability to support growth
(3) Biologic value (BV)—expression of the percentage of nitrogen retained for maintenance and growth compared with the amount absorbed
(4) Net protein utilization (NPU)—expression of the percentage of retained nitrogen compared with the amount ingested; differs from BV because it takes into account the protein’s digestibility
(5) Protein-digestibility-corrected amino acid score (PDCAAS)—compares the amino acid balance of a food protein with the amino acid requirements of preschool-aged children and then corrects for digestibility; used by the FDA for labeling
1. Primary—linear sequence of the component amino acids
2. Secondary—steric interaction of amino acids that are close to one another in the linear sequence (e.g., the α-helix and β-sheet)
3. Tertiary—steric interaction between amino acids that are far apart in the linear sequence, which causes folding and the ultimate functional structure of the protein (e.g., disulfide bonds)
4. Quaternary—steric interaction between subunits of proteins with more than one polypeptide chain (e.g., hemoglobin)
D Digestion, absorption, and transport (see Table 12-1 and Figure 12-1)
1. Mouth—mechanical breakdown and moistening
a. Hydrochloric acid from parietal cells denatures or unfolds proteins and activates pepsinogen to give pepsin
b. Pepsin begins the hydrolysis of the peptide bonds of proteins to form peptides and proteoses
a. The pancreas secretes bicarbonate into the duodenum to neutralize the acidic products from the stomach and proteolytic enzymes into an inactive form; enzymes activated by trypsin through a hormonal feedback mechanism are chymotrypsin, aminopeptidase, and carboxypeptidase; each hydrolyzes peptide bonds formed by different classes of amino acids
b. Enzymes of the brush border are dipeptidases that hydrolyze dipeptides to amino acids
4. Absorption—at the brush border of the microvilli of the small intestine, absorption occurs both by simple diffusion along a concentration gradient and by active transport at specific amino acid sites involving carrier enzymes, a sodium–ATP pump, and vitamin B6
5. Transport—absorbed amino acids collected by the portal blood system and transported to the liver
E Metabolism (see Figure 12-2)
1. Amino acid pool—a collection of amino acids in a dynamic equilibrium in the liver, blood, and other cells that provides the raw material for the body’s protein and amino acid needs
a. Input into the pool comes from proteins in the diet, breakdown of body proteins, and synthesis of nonessential amino acids
b. Output from the pool is for synthesizing body structures, specialized substances (e.g., melanin from tyrosine), and energy, as needed
a. De novo synthesis—requires deoxyribonucleic acid (DNA), messenger ribonucleic acid (mRNA), and ribosomal ribonucleic acid (rRNA)
(1) In the nucleus, DNA carries the genetic information in groups of three bases that provide the code for the individual amino acids comprising a specific protein
(2) mRNA transports a copy of the code from DNA into the cytoplasm
(3) mRNA attaches to a ribosome and acts as a template for the alignment of amino acids that are attached to transfer RNA (tRNA)
(4) If the proper amino acids are in the correct proportions and the synthetic enzymes and energy are available, the polypeptide chain is synthesized
3. Catabolism—amino acids in excess of those needed for the synthesis of proteins and other biomolecules cannot be stored or excreted; they may, however, be deaminated and the α-keto acid used as a metabolic fuel for immediate energy needs or for long-term energy storage as fat
(1) Deamination—loss of the α-amino group, usually in the liver, through transfer to α-ketoglutarate to form glutamate; glutamate is then oxidatively deaminated to yield ammonia (NH3)
(2) Urea cycle—series of steps whereby the ammonia produced during deamination is converted to urea for excretion
(1) Ketogenic amino acids are those whose carbon skeleton, after deamination, yields acetyl-CoA or acetoacetyl-CoA, which then yields ketone bodies; high concentrations of ketone bodies lead to some of the undesirable side effects of high-protein, low-carbohydrate diets, for example, ketoacidosis
(2) Glucogenic amino acids are those that yield pyruvate, α-ketoglutarate, and other intermediates of the citric acid cycle that can, if needed, be converted to glucose
4. Nitrogen balance—comparison measurement of the amount of nitrogen ingested with the amount excreted (e.g., urinary nitrogen plus approximately 1 g/day for nail, hair, skin, and perspiration losses) made to determine whether net protein catabolism, anabolism, or equilibrium exists
a. Positive balance—intake is greater than output; indicates net protein synthesis and is the normal situation for anyone building protein-containing tissue, such as during childhood, pregnancy, and recovery from undernutrition, surgery, or illness
b. Negative balance—intake is less than output; indicates net protein breakdown, when the body must break down its own protein to meet energy or metabolic needs; can result from insufficient protein (or essential amino acids) or energy intake or from fever, infection, anxiety, or prolonged stress
a. Anabolic—growth hormone, insulin, normal thyroid hormone, and sex hormones
b. Catabolic—adrenocortical hormones and large amounts of thyroid hormone
2. Vitamins—pyridoxine and riboflavin are necessary for protein synthesis; when they are deficient in the diet, synthesis may be limited
3. Energy source (4 kilocalories [kcal]/g)
4. Role of proteins in oral biology
a. Pre-eruptive effects on teeth—essential for all cells and therefore necessary for normal tooth bud and pulp formation and synthesis of protein matrix for enamel and dentin
1. Determination and estimates of protein requirements
a. Studies of nitrogen balance are used to determine the lowest protein intake that will support homeostasis or equilibrium
b. Average requirement for reference proteins of 0.8 g/kg/day for young adult males; other groups by extrapolation or interpolation
c. Estimates for growth needs in infants are based on the amount of protein provided by that quantity of human milk that ensures a satisfactory growth rate
2. Recommended dietary allowances—developed by the National Research Council; based on 1985 World Health Organization recommendations, which use nitrogen balance data; these allowances assume ingestion of good-quality protein in a mixed diet; adjustments are made for growth, pregnancy, and lactation
3. Food sources—protein needs of an average adult can be met by choosing two or more servings per day of meats, poultry, fish, eggs, dried beans, and nuts
I Dietary modifications for disease
a. Phenylketonuria (PKU)—inherited enzyme defect in which individuals cannot metabolize the phenylalanine found in nearly all proteins; the prescribed diet provides only enough phenylalanine to meet growth and maintenance needs; dietary protein is restricted, but amino acids are provided by a synthetic formula from which the phenylalanine has been removed
b. Other genetic disorders—maple syrup urine disease, homocystinuria, tyrosinemia, methylmalonic aciduria, propionic acidemia, and isovaleric acidemia are genetic disorders in which amino acid metabolism is altered; treated with low-protein diets and synthetic amino acid formulas
c. Gout—characterized by excessive uric acid production leading to the formation of urate crystals deposited in the joints; treatment often includes restriction of protein to limit purine and uric acid production
2. Protein needs are increased during fever, after severe injury and surgery, and by intestinal malabsorption, increased protein loss from the kidneys, or diminished protein synthesis by the liver
3. Dietary protein must be restricted when the kidneys can no longer remove nitrogenous wastes from the body or in severe liver disease when the nitrogenous byproducts of protein catabolism can no longer be synthesized
4. Protein-energy (calorie) malnutrition (PEM or PCM)
a. Kwashiorkor (classic)—failure of the young child to grow because of insufficient protein intake (usually following weaning from mother’s milk); edema often masks muscle wasting
b. Marasmus (classic)—failure of the infant or young child to grow because of partial starvation; total caloric and protein intakes are insufficient
c. Adult PEM or PCM—seen even in the developed countries among alcoholics and long-term hospitalized patients with acquired immune deficiency syndrome (AIDS), tuberculosis, and anorexia nervosa
Lipids (Fats)
A Definition—biochemical compounds composed of carbon, hydrogen, oxygen, and small amounts of phosphorus; insoluble in water and soluble in fatty substances and organic solvents
a. True fats—contain fatty acids attached to glycerol (a trihydroxy alcohol) through an ester linkage; these may be monoglycerides, diglycerides, or triglycerides, depending on the number of glycerol–hydroxyl groups esterified; chemical and biochemical characteristics of glycerides depend on the number, order, and kinds of fatty acids attached
(1) Saturated fatty acids—contain no double bonds and are found in lipids from animal sources; are solids at room temperature (high melting point)
(2) Unsaturated fatty acids—contain one or more double bonds and come from plant sources; are usually liquids at room temperature (low melting point)
(3) Hydrogenation—addition of hydrogen to some or all of the double bonds; used in the manufacture of margarine or butter substitutes from vegetable oils; in partial hydrogenation, some trans bonds are formed and may present a health risk
(4) Rancidity—addition of oxygen to some of the double bonds of fatty acids that contributes to spoilage; occurs spontaneously in foods and can be reduced by the addition of antioxidants, such as butylated hydroxytoluene (BHT)
(5) Iodine number—chemical indication of the degree of unsaturation of a fatty acid; the more molecules of iodine bound by the fatty acid, the more unsaturated and the higher is the iodine number
b. Waxes—esters of a fatty acid and an alcohol other than glycerol; the body is unable to use waxes because digestive enzymes do not hydrolyze their ester linkage
2. Compound lipids contain compounds added to the glycerol and fatty acids
a. Phospholipids (glycerol + 2 fatty acids + phosphate group = R group)
(1) Water-soluble emulsifiers (e.g., lecithin, with choline as the R group)
(2) Membrane constituents (e.g., sphingomyelin)
(3) Active intermediates in metabolism of lipid compound (e.g., CoA)
b. Glycolipids—contain a carbohydrate component and are found in the brain and nervous tissue (e.g., cerebrosides)
c. Lipoproteins—are water soluble and responsible for carrying lipids throughout the body
(1) Chylomicrons—approximately 2% protein; carry exogenous (absorbed from the diet) triglycerides around the body
(2) Very-low-density lipoproteins (VLDLs)—9% protein; carry endogenous triglycerides around the body
(3) Low-density lipoproteins (LDLs)—21% protein; carry mostly cholesterol from the liver to peripheral sites
(4) High-density lipoproteins (HDLs)—50% protein; carry cholesterol back to the liver; can be elevated by exercise
3. Derived lipids are compounds whose synthesis begins like fatty acid synthesis, with acetyl groups added on one at a time
a. Sterols—all have a polycyclic nucleus
b. Cholesterol is a precursor for the synthesis of many steroid compounds and a constituent of cell membranes
(a) Exogenous—average dietary intake is 400 to 600 milligrams (mg) from foods of animal origin
(b) Endogenous—average synthesis in the body is 1 to 2 g/day
(2) Regulation of cholesterol—dietary cholesterol, percentage of fat, ratio of polysaturated to monosaturated to unsaturated fat, and amount of certain fibers in the diet
c. Steroids—similar to sterols but with side-chain modification (e.g., bile acids, sex hormones, adrenocortical hormones, and vitamin D)
4. Artificial fats—substances developed for use in foods; have the flavor, appearance, and feel of dietary fats without their physiologic effects
a. Olestra—a zero-kilocalorie (0-kcal) artificial fat made from an indigestible combination of sucrose and fatty acids; may help serum cholesterol levels by directly interfering with cholesterol absorption; may increase the requirement for vitamin E; approved for use in snack foods
b. Simplesse—has approximately 15% of the kilocalorie of the fat it replaces; made by microparticulation of protein; the small protein particles have the feel of fat; not suitable for use in cooking but used in fat-free dairy products and salad dressings
c. Oatrim and maltodextrim—carbohydrate-based fat replacements; mimic the texture and feel of fat by forming gels; are digestible and contribute some calories
C Digestion, absorption, and transportation (see Table 12-1 and Figure 12-1)
a. Mouth—no enzymatic action; mechanical and moistening action only
b. Stomach—gastric lipase hydrolyzes some short-chain and medium-chain fatty acids from triglycerides
a. Short-chain fatty acids can be absorbed into the portal system
b. Medium-chain and long-chain fatty acids are water insoluble, require bile as a carrier (emulsifier), and are absorbed in stages
(1) Bile separated out at the intestinal wall and recirculated
(2) Complete breakdown of triglycerides within the mucosal cells by mucosal lipase
(3) Resynthesis of new triglycerides that combine with protein carriers to form chylomicrons
(4) Passage into the lymph system (lacteals) and blood through the thoracic duct
(5) At its destination, lipoprotein lipase hydrolyzes the triglycerides, clearing chylomicrons from blood
(6) Lipoprotein carriers (VLDLs, LDLs, and HDLs) carry endogenous lipids and cholesterol
D Metabolism (see Figure 12-2)
a. Lipogenesis—synthesis of triglycerides for long-term storage of energy; starting material is acetyl-CoA, which can come from glucogenic amino acids, carbohydrates, or breakdown of dietary lipids; lipogenesis takes place in nearly all cells but is most active in adipose cells
a. β-Oxidation—fatty acids are broken down in a stepwise manner to yield one molecule of acetyl-CoA for every two carbon atoms; acetyl-CoA can be catabolized further by means of the TCA and oxidative phosphorylation
b. Ketone production—when the body’s supply of carbohydrates is low, the TCA is depressed and acetyl-CoA from β-oxidation accumulates; alternative route for acetyl-CoA is ketone production; acetoacetone, acetone, and β-hydroxybutyrate are the ketone bodies; excess ketone production can cause ketosis, ketonuria, and ketoacidosis (which is sometimes fatal)
1. Vitamins as coenzyme precursors
a. Anabolism—biotin, riboflavin (in flavin adenine dinucleotide [FAD]), niacin (in nicotinamide–adenine dinucleotide [NAD]), and pantothenic acid (in CoA) (see Figure 12-2)
3. Enzymes necessary for the metabolism of lipids are synthesized or inhibited in response to the relative amounts of substrates and products available
F Biologic role and functions of lipids
1. Structural components of cell membrane
3. Carrier medium of fat-soluble vitamins
4. Protective padding for body organs
5. Insulation for the maintenance of body temperature
6. Role of lipids in oral biology
(1) Lipids provide a coating on the tooth’s surface and form a protective pellicle on the tooth.
(2) Lipids act by neutralizing the acids produced by bacterial metabolism of the plaque biofilm; they raise the pH and decrease risk of the demineralization of enamel.
b. No relationship between dietary fat and periodontal disease
1. Essential fatty acids (EFAs)—cannot be synthesized in sufficient amounts to meet the body’s needs; must be supplied in the diet; for humans the only EFAs are linoleic (ω-6) and linolenic (ω-3); requirement is approximately 3% of total kilocalories
a. Function—necessary for the synthesis of membranes and prostaglandins (local hormone)
b. Deficiency symptoms—seen in infants on low polyunsaturated fatty acid (PUFA) diets and in adults receiving total parenteral nutrition feedings without lipids; the deficiency is characterized by slow growth, reproductive failure, and skin lesions
2. Recommendations (dietary goals as recommended by the AHA)
a. Total fats—≤30% of total kilocalories; majority of calories should come from monounsaturated and polyunsaturated fatty acids
b. Cholesterol—≤300 mg/day; 200 mg/day for high-risk individuals
c. Saturated fats—avoid saturated and trans fatty acids (found in processed foods); less than 10% of total kilocuries should come from saturdated fatty acids
3. The seventh edition of Dietary Guidelines for Americans makes similar recommendations for healthy persons ages 2 years and older
H Dietary modifications for disease
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses