chapter 11
Pains of Dental Origin
In a survey of 45,711 American households, Lipton et al1 found that nearly 22% of the general population experienced at least one of five types of orofacial pain in the previous 6 months. The most common type of orofacial pain was toothache, which was reported by 12.2% of the population. Because toothache is so common, it is essential for the clinician who is managing orofacial pain to have a thorough understanding of its clinical presentation. Once the proper diagnosis is established, treatment for pains of dental origin is usually quite reliable.
The teeth are unique structures in that they are visceral tissues that function as part of the musculoskeletal system. This, perhaps, helps to explain some of the enigmatic behavior of dental pain. Tooth pulps serve as isolated organs, each an individual in its own right. The sensory capability of dental pulpal tissue is like that of other visceral structures, and pulpal pain is like other visceral pains. The connection of the tooth to the bone, however, is a true joint and therefore constitutes a musculoskeletal structure. This fibrous joint, called the periodontal ligament, is constructed in such a manner as to convert masticatory pressure into traction on the alveolar bone. These joints, termed gomphoses, are structurally similar to other fibrous joints in which traction forces predominate. The mechanoreceptors in periodontal ligaments are similar to those of other fibrous joints, although they differ considerably from the proprioceptors found in synovial joint ligaments, tendons, and muscles.2 Their nerve cell bodies are located in the mesencephalon, along with the other masticatory proprioceptors. They serve to inhibit the action of elevator muscles, which constitutes the driving force of masticatory function.3,4,5 The uniqueness of teeth and their alveolar attachment is that visceral structures function as an integral segment of the musculoskeletal system. The sensory behavior, including dental pain, constitutes a mixture of visceral and musculoskeletal characteristics (Fig 11-1).
It should be noted that while sensory input from the face is represented almost exclusively in the subnucleus caudalis, nociceptive signals from the oral cavity have double representation in the subnucleus oralis and caudalis.6 Periodontal afferent fibers terminate in the rostrally located main sensory trigeminal nucleus. The tooth pulp afferents, however, terminate there, as well as in all three subnuclei of the spinal tract of the trigeminal nucleus complex.7
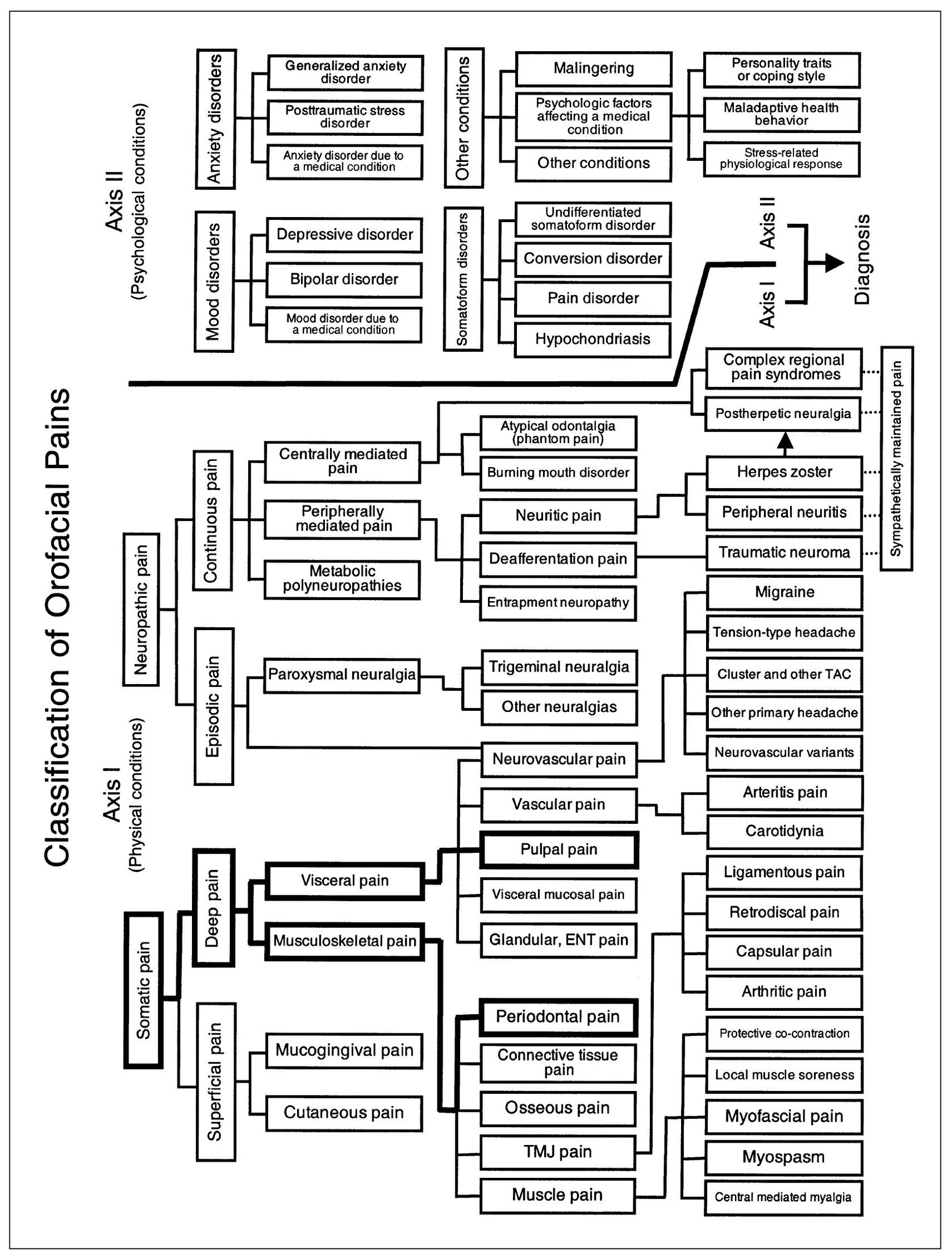
Fig 11-1 Pain classification chart highlighted to show the relationships of pulpal dental pain to other visceral pains and of periodontal dental pain to other musculoskeletal pains.
Dentin sensibility is mediated by way of the pulpal nerves. The intradental neural mechanism of this innervation is not yet known. Several theories have been offered: (1) the odontoblast-receptor theory, (2) the direct nerve ending theory, and (3) the hydrodynamic theory.8 It is known that many if not all nociceptive impulses from human dental pulps are conducted to the trigeminal nucleus by way of sympathetic visceral afferents that leave the head and reach the spinal cord through elements of the cervical sympathetic chain and upper thoracic spinal nerves.9
The unique innervation of teeth is pointed out by the report of two cancer patients who had undergone trigeminal tractotomy along with rhizotomy of the 9th and 10th cranial nerves and of several cervical spinal nerves to control intractable face pain. Both patients displayed analgesia or hypalgesia throughout the cutaneous trigeminal distribution; yet they continued to maintain normal sensory response to dental pulp stimulation.10
The sensory capabilities of the dental pulp are predominantly but not exclusively nociceptive.11 The dental pulp also senses thermal stimuli and tingling.12 It has been determined that, under specially controlled circumstances, pulpal receptors may give rise to nonnociceptive impulses that have some sensory function other than the mediation of painful sensation.13 It is thought that nonpainful sensations of the pulp are mediated by a distinct population of afferents that are nonnociceptive.14 The pulp contains both fast and slowly conducting neurons.15 There is conclusive evidence for the presence of a significant C-fiber population in tooth pulps that can be activated by electrical stimulation.16 The innervation density of the dental pulp is calculated to be about 15 times that of skin.17 The mediation of sensory impulses from the dental pulp to the subnucleus caudalis, as well as to the more rostrally located subnucleus oralis, has been confirmed.18,19
The teeth are united to the alveolar bone by fibrous joints, the periodontal ligaments comprising the flexible, fibrous portions. The collagenous fibers that connect the cementum to the alveolar cortex are arranged in an oblique fashion so as to convert the pressures of mastication into traction forces on the bone. The sensory receptors include free nerve endings and mechanoreceptors. When stimulated by pressure on the tooth, the mechanoreceptors exert an inhibitory influence on masticatory elevator muscle action.20,21 This response is known as the nociceptive reflex. It should be noted that sensory innervation of the teeth is strictly ipsilateral.22 Except for pain emanating from midface structures, sensory manifestations from the oral cavity are not expressed contralaterally or bilaterally.
Behavior of Dental Pains
It should be recognized that dental pains are extremely versatile and have the propensity to mimic nearly any pain disorder. The degree of such pain may vary from mild tenderness to unbearable intensity. Dental pain may be spontaneous, or it may be induced in various ways. It can be intermittent, with periods between attacks that are completely free of pain. It may be continuous but punctuated by lancinating exacerbations that radiate throughout the face and head. The extreme variability of toothache is such that a good rule for any examiner is to consider all pains about the mouth and face to be of dental origin until proven otherwise. An adequate dental examination is the essential first step in the management of all pain disorders involving the teeth, mouth, and face.
In spite of such variability, pains of dental origin present clinical features that categorize them as deep somatic pain. It is on the basis of these features that the examiner is able to clinically classify such pains.
Relationship of Dental Pain to Initiating Stimulus
The relationship between dental pain and noxious stimulation is accurate in that summation effects are not observed. Since pulpal receptors answer all noxious stimulation as pain, there may be considerable variation in threshold levels for different types of stimulation, such as thermal, electrical, and mechanical. Subthreshold stimulation is painless. The apparent delay between application of the irritant and pain reaction is not central summation, as seen in neuralgia, but rather represents the time required for the stimulation to reach threshold level.
Although accurate, the relationship between stimulus and pain reaction is not necessarily proportional. A moderate stimulus may induce pain that is disproportionate to it. Thus, the discomfort incurred does not indicate the seriousness of its cause.
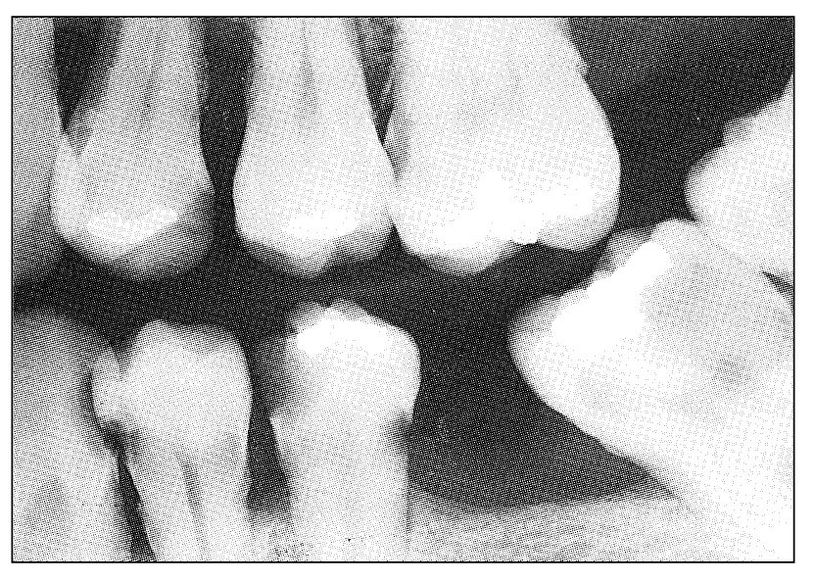
Fig 11-2 Radiograph showing rampant caries and extensive periodontal disease. Such a situation presents several possible causes of odontogenous pain, both pulpal and periodontal.
Types of Dental Pains
Dental pains may have their origin in the dental pulps or in the periodontal structures. These two categories will be discussed separately, since their clinical presentations are different.
Dental Pains of Pulpal Origin
The visceral character of pulpal pain is displayed particularly in its clinical behavior. The pain is of the threshold type, as compared with the graduated response exhibited by musculoskeletal pain. No response occurs until the threshold level is reached. Pulpal pain responds to noxious stimulation that is unrelated to ordinary masticatory movements. It responds to impact, shock, thermal and chemical irritants, and direct exploration, but not to ordinary masticatory function. The pain is often very difficult for the patient to localize.
Pulpal pains may be classified as acute, chronic, recurrent, or mixed with periodontal elements. A basic clinical feature of pulpal pain is that it does not remain the same indefinitely. Generally it resolves, becomes chronic, or proceeds to involve the periodontal structures by direct extension through the apex of the tooth root. Rarely does it remain unchanged for long periods.
Acute pulpal pain
Perhaps the most typical of all visceral pains is acute pulpal pain. It is often so difficult for the patient to localize that the patient may not be very helpful in determining the source of the pain. Objective evidence such as deep caries, erosion into the pulp chamber or root canal, or fracture or splitting may immediately identify the offending tooth. If such evidence is lacking, and especially if more than a single tooth might be involved (Fig 11-2), clinical identification of the offending tooth may be difficult, if not impossible.
The cause of acute pulpal pain is noxious stimulation of the pulpal receptors. Normally, the tooth structure protects these nerve endings from superficial stimulation, so that only extreme surface irritation such as electric stimulation or excessive thermal change is sensed as pain. If the tooth structure is breached by splitting, a normal pulp may immediately become painful on contact with saliva or air. This occurs especially when masticatory stress tends to open the split. If the tooth structure is breached by fracture of a submerged metallic filling, pain results from pressure exerted against the pulp and is increased when additional pressure is brought to bear on the filling by biting against it. Until the pulp becomes inflamed, pain from such cause is intermittent.
The pulp tissue responds to injury, whether it be from repeated threshold stimulation of the intact surface, cervical exposure due to gingival recession, repeated irritation of occlusal trauma, breaching of the overlying protective tooth structure due to splitting or fracture, repeated thermal shocks transmitted by metallic fillings, thinning of the overlying tooth structure by erosion or abrasion, traumatic shock, or dental caries. The changes that take place are usually inflammatory. These conditions may be reversible unless congestion occurs and causes pulpal necrosis or gangrene. Once tissue death occurs, extension to the periodontal tissue through the apical foramen of the root almost invariably takes place. This is especially so if the pulpal inflammation is the result of bacterial agents. It should be noted that in multirooted teeth irreversible pulpal changes may occur in one root with reasonably normal pulp tissue surviving in another root. Situations of this type may not occur frequently, but when they do, a confusing symptom picture may result.
It is crucial to understand the pulpal pains that result from such tissue changes. As a rule, the pain threshold of all deep receptors and nerve fibers that mediate pain is lowered by inflammation. Thus, the inflamed dental pulp is hypersensitive to all stimuli, including electric stimulation, thermal shock, probing, and percussion. Pain may be elicited by the application of some or all such stimuli to the tooth. If the tissue change is not too great, application of such stimuli causes pain only for the duration of the noxious stimulation. But if the change is greater, such stimulation may induce a prolonged toothache. As the inflammatory process progresses, spontaneous toothache may occur with no outside provocation.
Acute pulpal pain may range from occasional hypersensitivity caused by sweets and other minor stimulants to spontaneous, violent, throbbing toothache of intolerable intensity that cannot be controlled even with narcotic analgesics. It may be induced by many types of irritants or be wholly spontaneous. It may be increased by both heat and cold or increased by heat and relieved by cold. It may be intermittent or continuous. It may relate to the contact of teeth or to the presence or temperature of foods and beverages. It may be mistaken for sinus headache, sinusitis, earache, neuritis, trigeminal neuralgia, or atypical facial pain. It may induce referred pains, areas of secondary hyperalgesia almost anywhere in the face and head, localized autonomic symptoms, and secondary painful masticatory muscle co-contraction. It may induce a masticatory muscle disorder or be mistaken for temporomandibular joint arthritis. If the pulpal inflammation is great enough to cause severe, continuing toothache, the chance of resolution is extremely low. Under favorable circumstances, resolution does occur. Usually the condition progresses to pulpal necrosis. When this occurs, pain from the pulpal tissue ceases. Such “recovery” from severe toothache should be interpreted as the transition period from pulpal to periodontal involvement. This transition is extremely variable. If the pulpal inflammation is the result of infection, the transition is usually rapid and consists of mixed pulpal and periodontal pains at first, terminating as an acute periodontal abscess (Fig 11-3). If the pulpal inflammation is sterile, all pain may cease, and a painless periapical granuloma or radicular cyst may develop (Fig 11-4). All gradations between these extremes may occur. If a multirooted tooth is involved, one root may exhibit symptoms of acute pulpitis, whereas another may show evidence of pulpal necrosis and periapical abscess (Fig 11-5). When this occurs, the symptom picture can become very confusing.
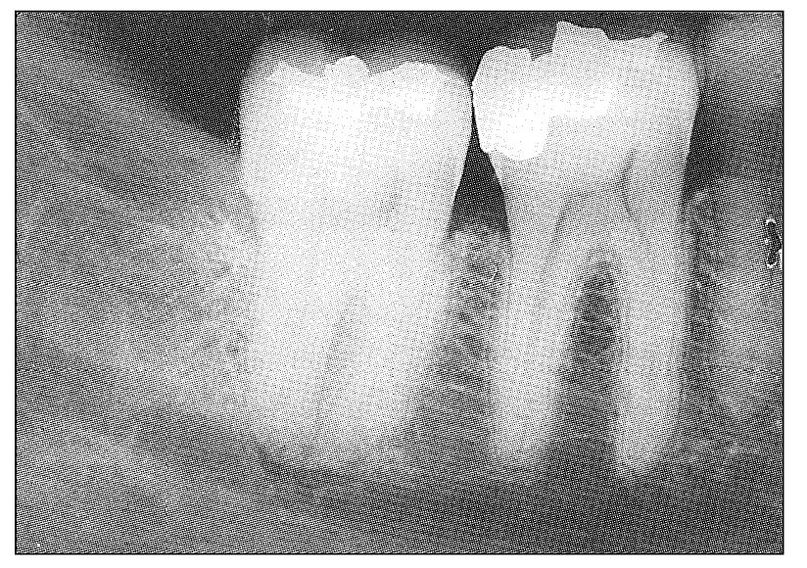
Fig 11-3 Radiograph showing early periapical radiolucent change at the mandibular second molar indicative of periapical abscess. This tooth was split mesiodistally and had elicited recurrent acute pulpal pains on several occasions. As the periapical tissues became involved, both pulpal and periodontal pain could be identified. Early in its clinical course, the first molar was suspected because of its large amalgam restoration.
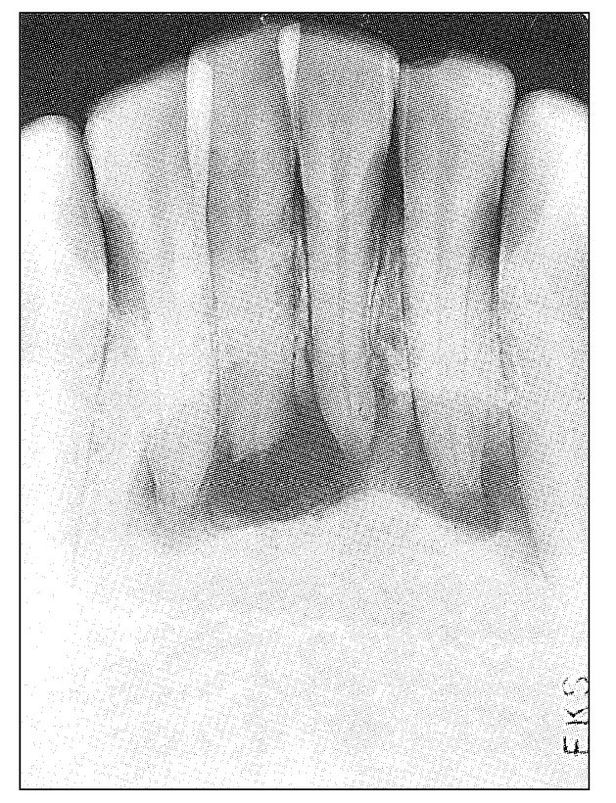
Fig 11-4 Radiograph showing an extensive apical radiolucency involving the mandibular anterior teeth. The four incisors were slightly discolored and nonresponsive to thermal and electric stimulation. The condition was painless. The history was that of former trauma and acute pulpal pain that soon subsided.
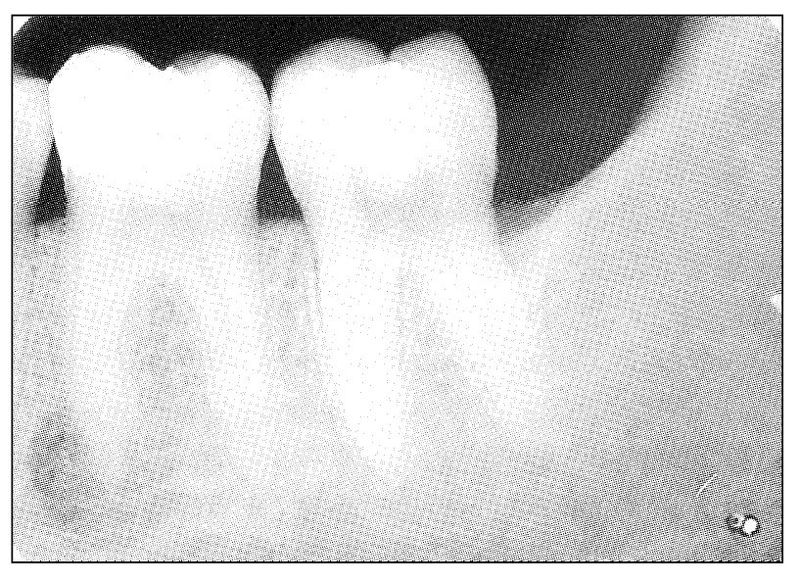
Fig 11-5 Radiograph showing periapical radiolucent change involving the mesial root of the mandibular first molar tooth. Clinical symptoms were those of typical pulpal pain and acute periodontal pain occurring simultaneously in the tooth.
Chronic pulpal pain
Under certain conditions, injured pulpal tissue may progress from an acute to a chronic inflammatory phase and thus undergo changes that proceed neither to resolution nor to necrosis but remain indefinitely as what is usually described as chronic pulpitis. The condition that favors this transition is traumatic injury to a young tooth, especially one with an open, expansive root end that is less likely to facilitate congestion and necrosis of the pulp. Such teeth continue to respond to pulp testing, although they are considerably less responsive than those with normal pulps. Frequently, internal resorption of the tooth occurs (Fig 11-6).
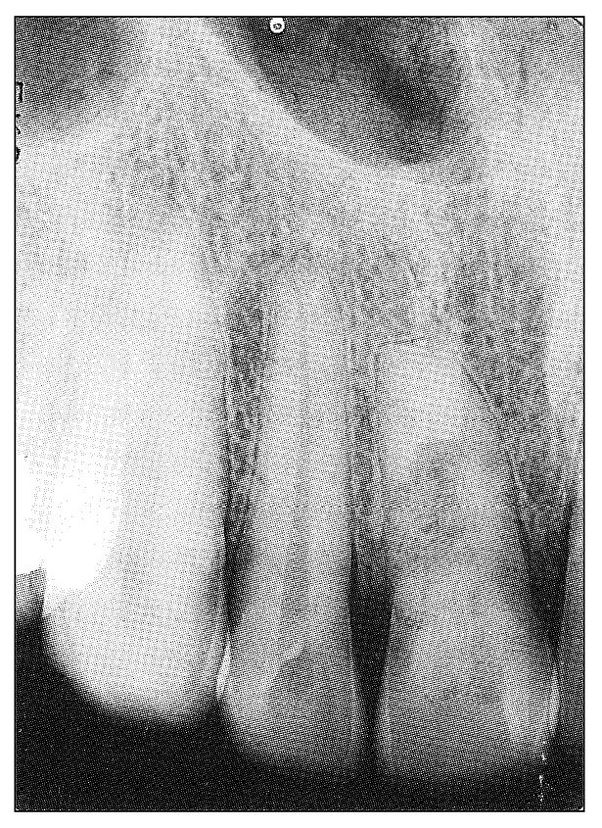
Fig 11-6 Radiograph showing central radiolucency in the maxillary central incisor that is indicative of internal resorption. The tooth had a normal color and responded positively to electric pulp testing but considerably less than the adjacent teeth. The patient was a 32-year-old man. There was history of trauma to his tooth 16 years previously, followed by a “toothache” that promptly subsided. Presently the tooth is asymptomatic.
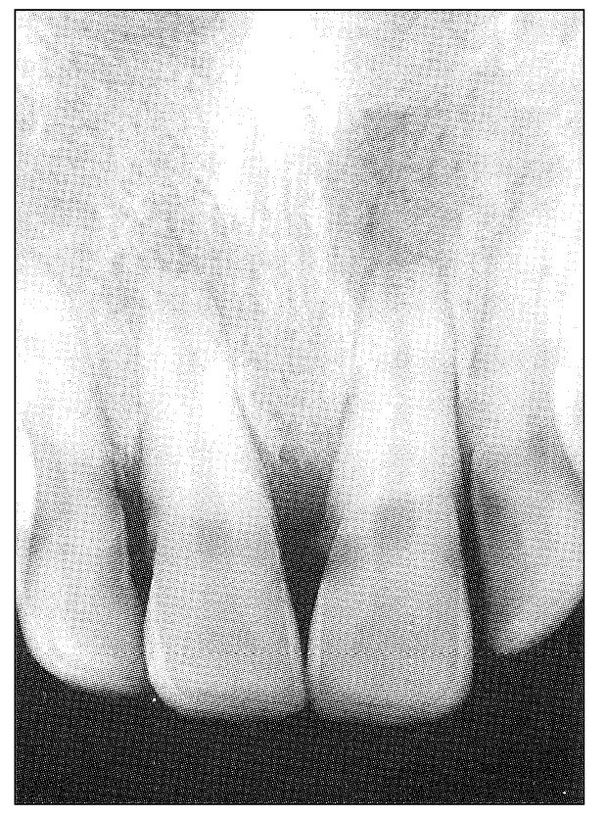
Fig 11-7 Radiograph showing apical and periapical radiolucency of the maxillary central incisors. Both had a normal color and responded positively to electric pulp testing but considerably less so than the adjacent teeth. Both teeth, especially the right incisor, were described as vaguely uncomfortable but not painful.
When chronic pulpitis develops, the pain responses change from the extremely variable character of acute pulpal pain to a milder and less variable discomfort, which may not be described as pain at all (Fig 11-7). In fact, the tooth may become asymptomatic unless further injury to it takes place.
Recurrent pulpal pain
Severe acute pulpal pain is rarely recurrent in the true sense because the inflammation usually progresses either to resolution, chronicity, or necrosis. When acute pulpal pain appears to be recurrent, it usually consists of recurring periods of inflammation in a sequential pattern. This may occur when a partially split tooth is opened only by some unusual occlusal stress. Ordinarily, true recurrent pulpal pain does not cause a toothache but is sensed as recurrent hypersensitivity. Such conditions are frequently associated with changes in vascular pressure or fluid balance. So-called menstrual toothache and high-altitude toothache fall into this category. The offending tooth is usually slightly inflamed, and the lowered pain threshold becomes symptomatic when the proper environmental conditions prevail. Other instances of recurrent pulpal pain include hypersensitive teeth that are stimulated to the point of pain by such factors as sweets, thermal changes, and occlusal abuse.
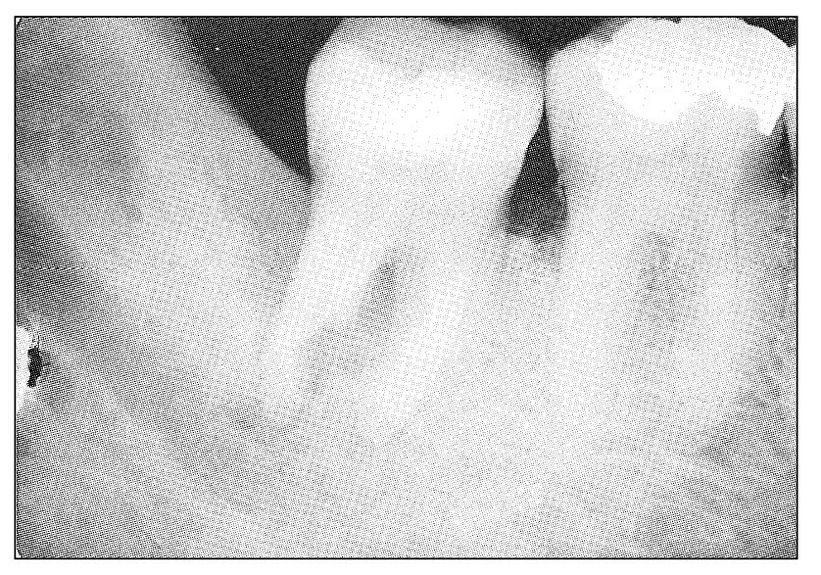
Fig 11-8 Radiograph showing periodontal radiolucency involving the distal root of the mandibular second molar tooth. There is extensive interradicular involvement and deep surface resorption into the mesial aspect of the distal root. Clinically, the tooth had symptoms indicative of a chronic periodontal abscess, with more recent symptoms of acute pulpitis. The tooth was responsive to electric pulp testing.
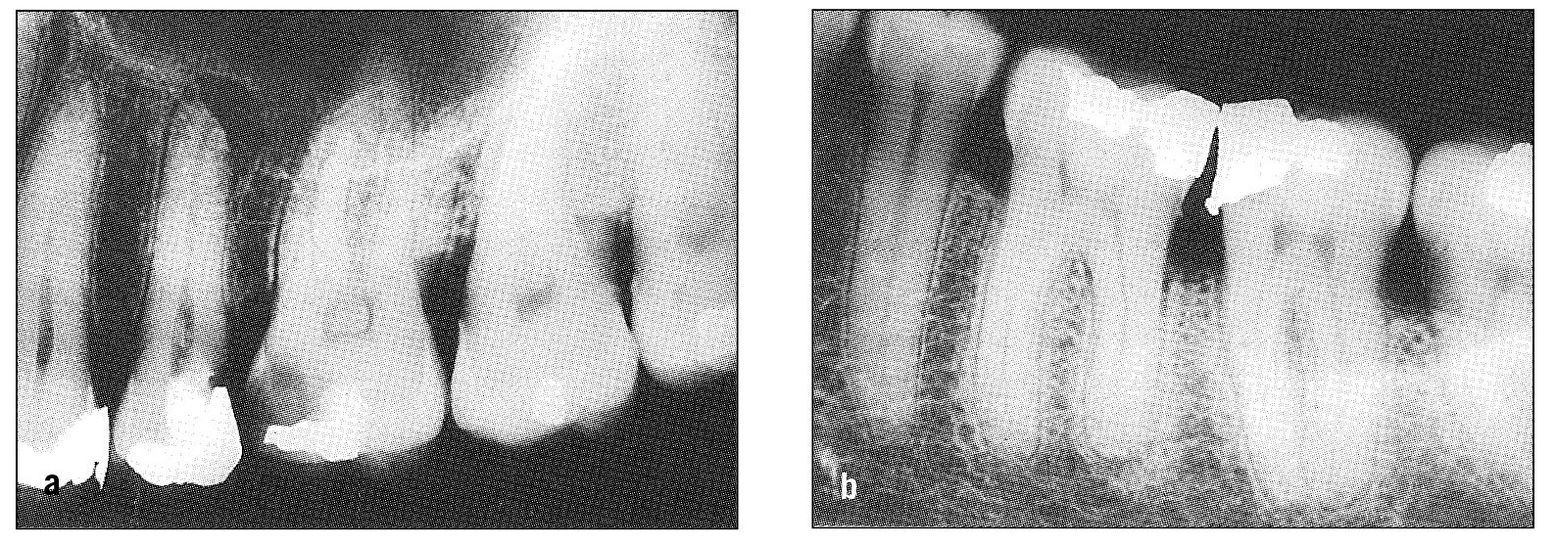
Fig 11-9 Radiographs showing reasonable cause for pulpal and periodontal pain in adjacent teeth. (a) The maxillary second premolar shows periapical radiolucency; the first molar has extensive dental caries. (b) There is evidence of recurrent caries beneath the mesial filling in the mandibular second molar; the distal root of the first molar shows slight but definite periapical involvement.
When the transition from pulpal inflammation through necrosis to periapical involvement is rapid, symptoms of both pulpal and periodontal pain may occur. Conversely, when the dental pulp becomes secondarily involved by direct extension from the periodontal structures, mixed symptoms may be evident (Fig 11-8). Also, especially as a result of trauma, both pulpal and periodontal symptoms may occur simultaneously. In all such instances, the cause is usually obvious and the symptoms readily explained. However, a tooth can present with pulpal and periodontal symptoms arising from separate and independent causes, in which case the symptom picture may be confusing. This can occur especially in multirooted teeth. Also, nearby teeth may be simultaneously involved and have symptoms that are misleading to examiner and patient alike (Figs 11-9a and 11-9b).
Obscure causes of pulpal pain
There are several fairly common causes of pulpal pain that are clinically difficult to recognize. Perhaps the most common of these is the mesiodistally split tooth, the margins of which are obscured by adjacent teeth and the presence of which is not radiographically visible. Another common cause is an occlusal filling that is slightly dislodged in a pulpal direction. Recurrent caries obscured both clinically and radiographically by an overlying dental restoration may be the etiologic factor. Still another cause is mechanical abrasion of the root of a tooth due to the presence of an adjacent impacted tooth (Fig 11-10). Abrasions can penetrate the substance of the root until the contents of the root canal are exposed. Another very obscure cause of pulpal involvement is direct extension from a periodontal pocket via an aberrant lateral root canal. Hematogenous infection of the dental pulp has been reported.
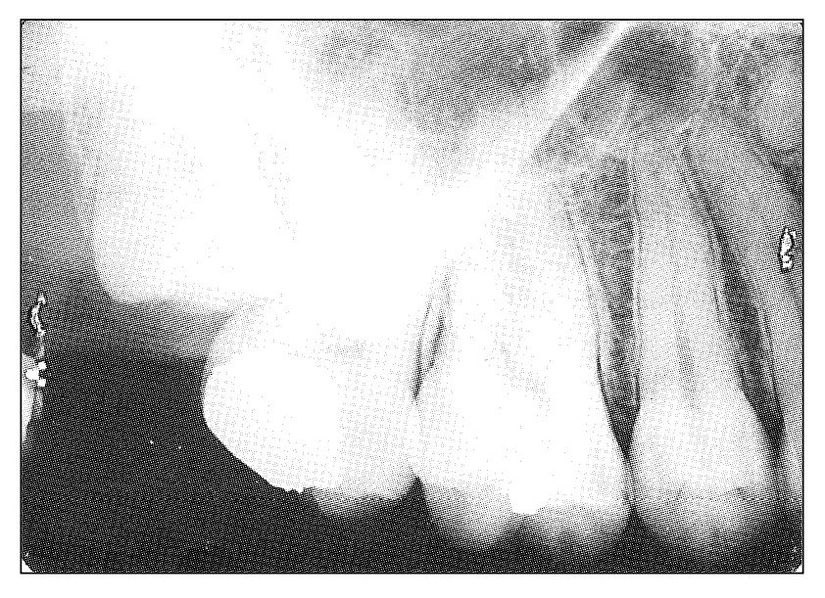
Fig 11-10 Radiograph of an impacted maxillary third molar that had abraded into the root of the second molar. The patient presented with symptoms of acute pulpal pain emanating from the second molar. The tooth was sacrificed. The abraded area involved the nerve canal of the distobuccal root.
All such possibilities should be considered and explored when pain appears to be truly odontogenous but the cause is obscure. Occasionally, direct exploration of the tooth is justified if the pain is severe and the patient is unwilling to wait for natural localization. The risk of opening an innocent dental pulp may be justified to establish a positive diagnosis. This is better than the more radical approach, for example, of extraction of such a tooth on a presumptive diagnosis. At least with the former treatment, endodontic measures can be used to salvage the tooth if a mistake is made.
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


