Odontogenic Tumors
Several classification schemes based on histologic patterns have been devised for this complex group of lesions. Common to all is the division of tumors into those composed of odontogenic epithelial elements, those composed of odontogenic mesenchyme, and those that are proliferations of both epithelium and mesenchyme (ectomesenchyme). As classified on the basis of biological behavior, they range from clinically trivial (i.e., benign, no recurrence potential) to malignant (Box 11-1).
Epithelial Tumors
Ameloblastoma
Pathogenesis
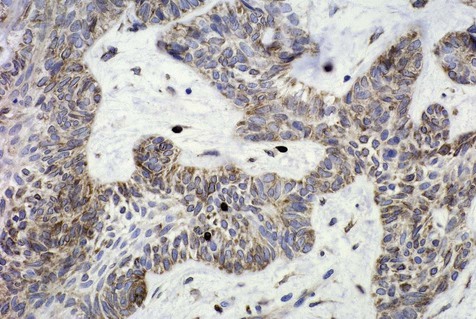
Mechanisms by which ameloblastomas gain a growth and invasion advantage include those associated with tumorigenesis and differentiation as well as other molecules related to tumor progression. These include but are not limited to overexpression of tumor necrosis factor (TNF)-α, antiapoptotic proteins (Bcl-2, Bcl-xL), and interface proteins (fibroblast growth factor [FGF], matrix metalloproteinases [MMPs]) (Figure 11-1 and Box 11-2). Ameloblastomas, however, have a low proliferation rate, as shown by staining for the cell cycle–related protein, Ki-67. Suggestions that dysregulation of the sonic hedgehog (SHH) pathway may play a role in the development of ameloblastoma have been put forth, although it must be emphasized that the overall significance of this is unclear. Mutations of the p53 gene do not appear to play a role in the development and growth of ameloblastoma; a role for ameloblastin protein has been identified, although it is not specific to ameloblastoma.
Clinical Features
Ameloblastoma is chiefly a lesion of adults. It occurs predominantly in the fourth and fifth decades of life, and the age range is very broad, extending from childhood to late adulthood (mean age, approximately 40 years) (Box 11-3). The rare lesions occurring in children are usually cystic and appear clinically as odontogenic cysts. There appears to be no gender predilection for this tumor.
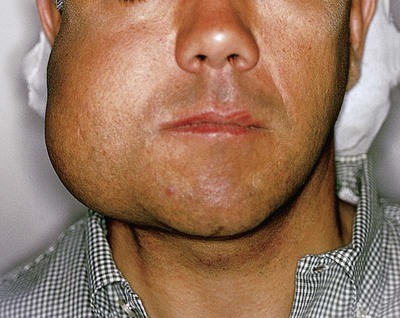
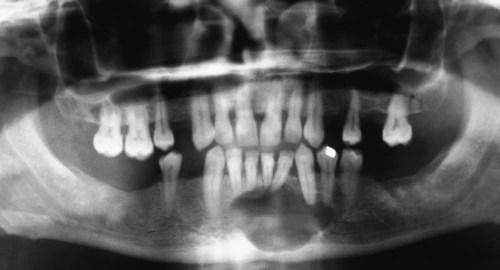
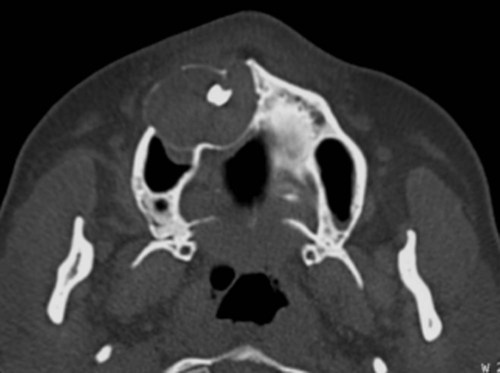
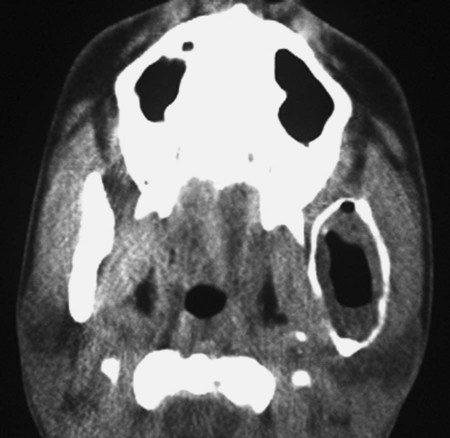
Ameloblastomas may occur anywhere in the mandible or maxilla, although the mandibular molar-ramus area is the most common site. In the maxilla, the molar area is more commonly affected than the premolar and anterior regions. Lesions usually are asymptomatic and are discovered during routine radiographic examination or because of asymptomatic jaw expansion (Figures 11-2 and 11-3). Occasionally, tooth movement or malocclusion may be the initial presenting sign.
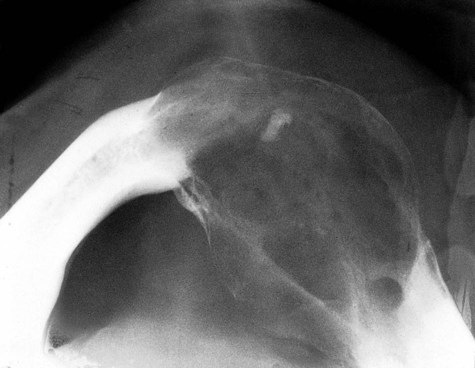
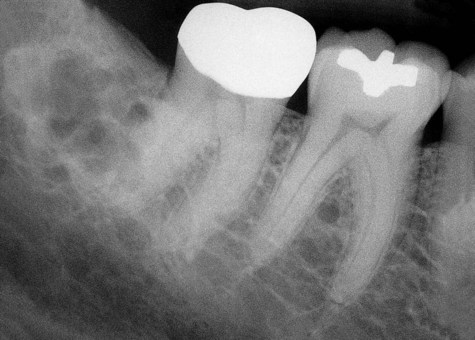
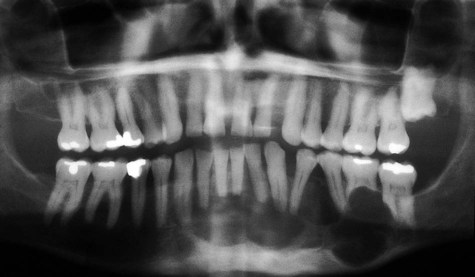
Radiographically, ameloblastomas are osteolytic, typically found in the tooth-bearing areas of the jaws, and they may be unicystic or multicystic (Figures 11-4 to 11-7). Because ameloblastomas are slow-growing, the radiographic margins usually are well defined and sclerotic. In cases in which connective tissue desmoplasia occurs in conjunction with tumor proliferation, ill-defined radiographic margins are typically seen. This variety, known as desmoplastic ameloblastoma, also has a predilection for the anterior jaws and radiographically may resemble a fibro-osseous lesion. The generally slow tumor growth rate may be responsible for the movement of tooth roots. Root resorption occasionally occurs in association with ameloblastoma growth.
Biological Subtypes
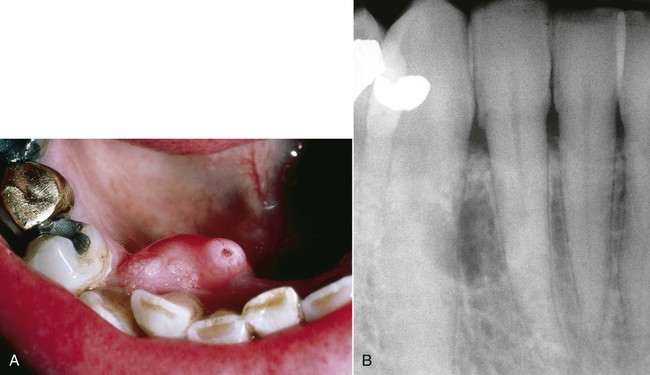
Peripheral or extraosseous ameloblastomas may occur in the gingiva and very rarely in the buccal mucosa (Boxes 11-4 and 11-5; Figure 11-8). These lesions are seen in older adults, usually between 40 and 60 years of age. They may arise from overlying epithelium or rests of Serres. They exhibit a benign, nonaggressive course and generally do not invade underlying bone. Following local excision, recurrence is rare.
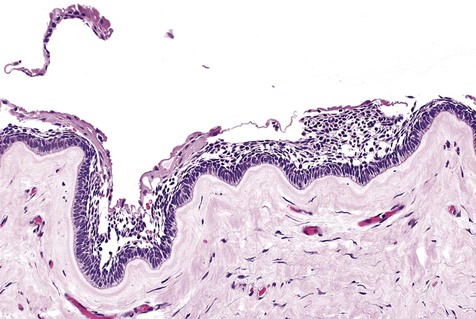
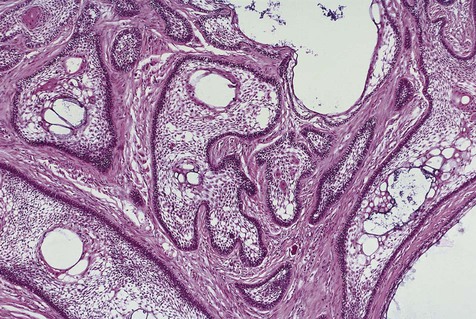
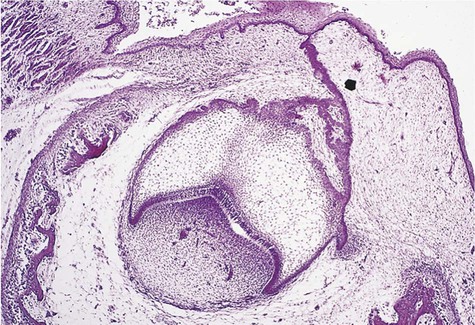
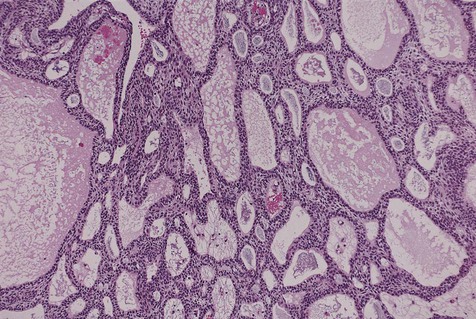
Cystic ameloblastoma (also referred to as unicystic ameloblastoma) accounts for approximately 6% of ameloblastomas. We prefer the term cystic ameloblastoma because these entities are often multilocular, show cortical perforation in 25% of cases, and have a recurrence rate as high as 40% when treated by curettage (as late as 9 years following surgery) (Box 11-6; Figures 11-9 and 11-10). They are seen in a younger age group (mean age, ≈35 years) than solid tumors. The microscopy is deceptive because the lesions are nearly completely cystic and can be confused with a simple odontogenic cyst (Figures 11-11 and 11-12).
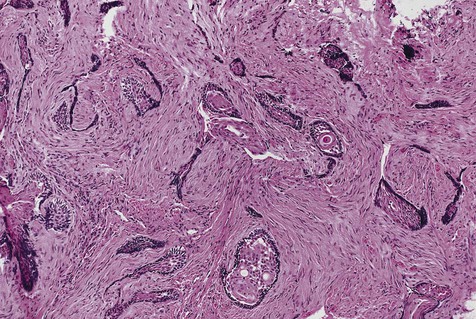
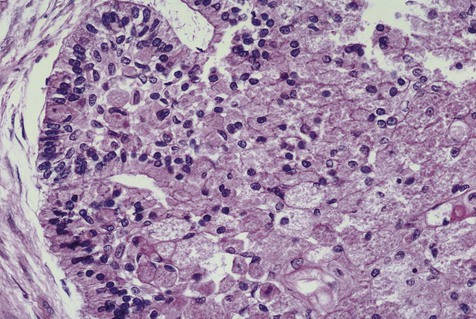
Malignant variants of ameloblastomas may rarely be encountered. These lesions occur in a relatively young age group (thirties) and appear in the mandible more commonly than in the maxilla. By definition, these are lesions that metastasize to local lymph nodes or distant organs. Direct extension into contiguous areas does not qualify for a malignant designation. Malignant lesions have been divided into two subtypes: malignant ameloblastoma (Figure 11-13), in which primary and metastatic lesions are microscopically well differentiated with the characteristic histologic features of ameloblastoma, and ameloblastic carcinoma (Figure 11-14), in which the lesions (primary and/or metastatic) exhibit less microscopic differentiation, showing cytologic atypia and mitotic figures. Malignant variants of ameloblastomas are difficult to control locally. Metastases may appear, usually in the lung, as a result of aspiration of tumor cells or by hematogenous spread after multiple unsuccessful attempts at primary tumor control. Regional lymph nodes are the second most common metastatic site, followed by the skull, liver, spleen, kidney, and skin.
Histopathology
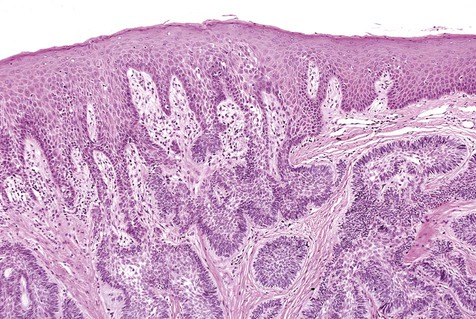
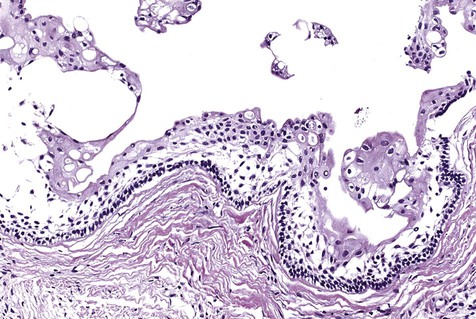
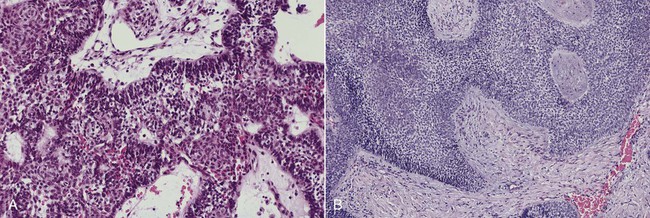
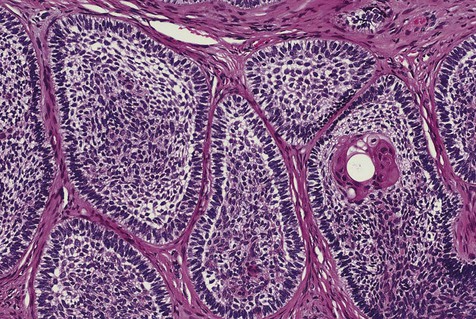
The numerous histologic patterns described for ameloblastoma are of no clinical relevance (Box 11-7). Some may exhibit a single histologic subtype, and others may display several histologic patterns within the same lesion. Common to all subtypes is the palisading of columnar cells around epithelial nests in a pattern similar to that of ameloblasts of the enamel organ. Central to these cells are loosely arranged cells that mimic the stellate reticulum of the enamel organ (Figure 11-15). Another typical feature is the budding of tumor cells from neoplastic foci in a pattern reminiscent of tooth development.
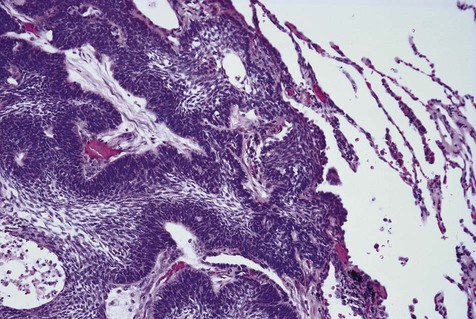
The microscopic subtype most commonly seen in solid ameloblastoma is the follicular type (Figure 11-16). It is composed of islands of tumor cells that mimic the normal dental follicle. Central cystic degeneration of follicular islands leads to a microcystic pattern (Figure 11-17). Neoplastic cells occasionally develop into a network of epithelium, prompting the term plexiform ameloblastoma (Figure 11-18). When the stroma is desmoplastic and the tumor islands become squamous appearing (squamoid) or elongated, the term desmoplastic ameloblastoma is used (Figure 11-19). Some tumors are microscopically similar to basal cell carcinoma and are called basal cell or basaloid ameloblastomas. A type of solid ameloblastoma in which the central neoplastic cells exhibit prominent cytoplasmic granularity (and swelling) is known as granular cell ameloblastoma (Figure 11-20). Clear tumor cells and cells expressing ghost cell–type keratinization have also been seen in ameloblastomas. Separation of ameloblastomas into the various microscopic groups described is essentially an academic exercise, because there appears to be no correlation between clinical behavior and these microscopic patterns.
Calcifying Epithelial Odontogenic Tumor (Pindborg Tumor)
Calcifying epithelial odontogenic tumor (CEOT), also known as Pindborg tumor after the oral pathologist who first described the entity, is a benign tumor of odontogenic origin that shares many clinical features with ameloblastoma (Box 11-8). Microscopically, however, there is no resemblance to ameloblastoma, and radiographically distinct differences will often be noted. The cells from which these tumors are derived are unknown, although dental lamina remnants and the stratum intermedium of the enamel organ have been s/>
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses