11
Local anaesthetics
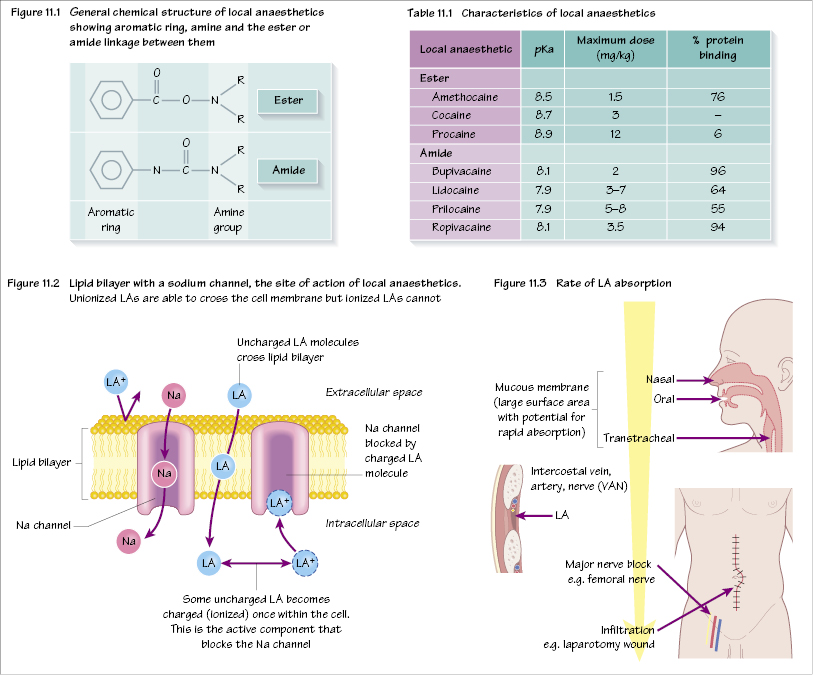
Local anaesthetics (LAs) are weak bases. They are divided into two groups, depending on the linkage between an aromatic ring and an amine group (Table 11.1). This linkage is either an amide or an ester (Figure 11.1).
Esters
(e.g. cocaine, procaine, amethocaine) Allergic reactions are common. Metabolized by plasma and liver cholinesterase.
Amides
(e.g. bupivacaine, lidocaine, ropivacaine) Allergic reactions are rare. Metabolized by the liver.
Uses
The uses of LAs include:
- local infiltration, e.g. laceration suturing, postoperatively to surgical wounds;
- topical to mucous membrane, e.g. cornea, nose, oropharynx;
- spinal cord – epidural and spinal;
- minor nerve blockade, e.g. radial nerve;
- major nerve trunk blockade, e.g. brachial plexus block;
- intravenous regional anaesthesia – Bier’s block with prilocaine or lidocaine;
- cardiac tachydysrhythmias, e.g. lidocaine for ventricular tachycardia (VT);
- topical to skin – eutectic mixture of local anaesthetics (EMLA), amethocaine;
- reducing discomfort of propofol injection, e.g. by adding 10 mg lidocaine.
Mechanism of action
LAs act by reversible inhibition of action potential transmission in all excitable tissues. They block sodium channels of nerve cell membranes and prevent sodium influx and action potential propagation and hence nerve conduction (Figure 11.2). Only the non-polar (lipophilic) form of the drug can cross the cell membrane and, once intracellular, the polar component becomes the active drug, which blocks the channel. The higher the frequency of sodium channel opening, the more susceptible is the nerve to blockade – hence sensory nerve fibres are blocked before motor nerves.
Speed of onset of action
This is related to the amount of drug in the unionized form that can cross the cell membrane. This depends on its pKa (pH at which 50% of the drug is in the ioni/>
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


