Diseases of Salivary Glands
Carol M Stewart, Indraneel Bhattacharyya, Madhu K Nair, James C Pettigrew, Seunghee Cha and Joseph Katz
Developmental Disturbances
Aplasia/Agenesis and Related Aberrancy of Salivary Glands
Congenital aplasia or agenesis of the major salivary glands is an uncommon finding, characterized by partial or total lack of development of the gland. Agenesis may involve one gland, pairs, or multiple glands, and be unilateral or bilateral. It may be a single independent finding or be associated with other developmental anomalies.
Hyperplasia of Minor Salivary Glands
Histopathology
Histopathologic examination demonstrates lobular aggregates of normal appearing mucous acini that are greater in number (hyperplasia) than normally would be found in the area. These glands may appear to be increased in size (hypertrophy). Chronic inflammation, if present, is usually mild and localized. Shimoyama et al reported that Ki-67 staining for cell proliferative activity demonstrated no statistically significant differences among adenomatoid hyperplasia and a matched group of normal palatal salivary glands. The conclusion was that adenomatoid hyperplasia had limited growth potential.
Saliva, Xerostomia, Hyposalivation and Sialorrhea
Saliva
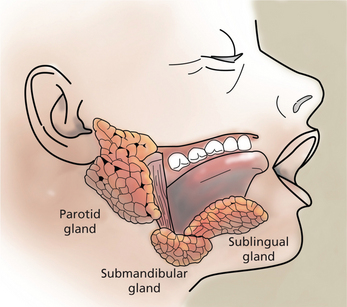
Saliva is an essential fluid for maintaining oral and systemic health and a satisfactory quality of life. While saliva is sometimes considered bothersome during restorative procedures, the alteration in quality and/or quantity of saliva has serious sequelae for the patient. Saliva is the product of three paired major salivary glands (the parotid, submandibular, and sublingual), and minor glands found in the hard and soft palate, lips, tongue, and buccal mucosa (Figure 1). Major salivary glands are composed of ductal and acinar structures. The acinar cells are the secretory components and ductal cells are the branching network that transports saliva into the oral cavity. The acinar cells of the parotid gland are purely serous elements and upon stimulation, parotid secretions account for at least half of the volume of whole saliva. Serous secretions are ‘watery’ and contain digestive enzymes, water, salts, and ions. They aid in mastication, clearing agents from the oral cavity, and facilitate swallowing and speech. Fluid from the parotid gland enters the oral cavity through the main excretory duct, Stensen’s duct, located in the buccal mucosa next to the maxillary second molars. In the submandibular gland, the acinar components are mixed mucous-serous, but predominantly serous. At rest, the submandibular gland secretions account for approximately two-thirds of the unstimulated saliva production. Wharton’s duct is the main duct of the submandibular gland and enters the floor of the mouth on either side of the lingual frenum. The sublingual gland, the smallest of the three major glands, is located above the mylohyoid muscle. Acinar elements are mixed, but predominantly mucous. The mucin produced facilitates lubrication and swallowing. The main excretory duct of the sublingual gland, Bartholin’s duct, may join Wharton’s duct resulting in a blending of secretions, or open into the oral cavity with a separate sublingual papilla. Numerous sublingual ducts may join the submandibular gland duct or open separately into the floor of the mouth. Minor salivary glands are named according to their location—lingual (anterior and posterior), labial, buccal, palatine and glossopalatine. Table 1 summarizes the nomenclature for the major and minor salivary glands and their acinar components.
Table 1
Major and minor salivary glands and secretion
| Glands | Type of secretion |
| Parotid | Purely serous |
| Submandibular | Mixed, however predominantly serous |
| Sublingual | Mixed, however predominantly mucous |
| Lingual minor salivary glands | |
| Anterior lingual (glands of Blandin and Nuhn) | Primarily mucous Purely serous |
| Posterior lingual (glands of von Ebner) | Purely mucous |
| Labial and buccal minor salivary glands | Mixed |
| Glossopalatine and palatine minor salivary glands (Weber’s glands) | Purely mucous |
In health, approximately 750 ml of saliva is produced in a day. The principal control of salivary secretion is mediated by sympathetic and parasympathetic innervation. The adrenergic (α and β) receptors are regulated by the sympathetic nervous system and the muscarinic receptors by the parasympathetic nervous system. At least five muscarinic-cholinergic receptor subtypes exist and it has been determined that the muscarinic receptor that medicates secretion of saliva is the M3 subtype. The parasympathetic nerve stimulation leads to an increased volume of saliva, whereas the sympathetic stimulation has an effect on protein content and salivary composition.
Hypofunction and Xerostomia
Dry mouth has a variety of causes as indicated in Box 1. Common causes of salivary gland dysfunction leading to hyposalivation include medications, irradiation to the head and neck, and autoimmune diseases such as systemic lupus erythematosus (SLE) and Sjogren’s syndrome (SS). Other systemic conditions affecting salivary glands include sarcoidosis, sialadenosis, and viral infections such as HIV and hepatitis C.
Mouth-breathing, tobacco smoking, alcohol use, and consumption of beverages containing caffeine can contribute to oral dryness as well. Age-related hyposalivation has been reported due to structural changes in salivary glands with increasing age. Other studies have reported no age-dependent decrease in saliva flow rate in healthy elderly populations. The incidence of xerostomia will increase and the oral manifestations will continue to be a challenge for the clinician.
Xerostomia and Salivary Gland Hypofunction due to Medications
A study of medications and caries among older individuals by Thomson et al revealed that an adjusted coronal caries increment (AdjCI) was higher among males and those taking a beta blocker or an anti-asthma drug for the previous 5 years. Several classes of drugs have been associated with dry mouth (Box 2). The most common groups include those with: (i) anticholinergic effects such as tricyclic antidepressants, drugs for urinary retention and over-active bladder, antipsychotics, diuretics, and antihistamines; (ii) sympathomimetic drugs such as antihypertensives, anti-depressants, decongestants, and bronchodilators; (iii) skeletal muscle relaxants; (iv) benzodiazepines; (v) proton pump inhibitors; and (vi) anti-migraine agents. Dry mouth has been reported to be a consequence of cytotoxic drugs such as 5-fluorouracil. Medications used for treatment of HIV have also been associated with dry mouth. These include didanosine and protease inhibitors.
Sjögren’s Syndrome
Sjögren’s syndrome occurs worldwide and while it may occur at any age, the peak incidence is between 40 and 50 years. Sjögren’s syndrome has one of the highest female-to-male ratio (9:1) of any autoimmune rheumatic disease. In addition to ocular and oral dryness, a wide spectrum of extraglandular manifestations may occur as well. The musculoskeletal, hematological, vascular, pulmonary, gastrointestinal, dermatological, renal and nervous systems may be involved. Patients with SS have an increased risk of developing lymphoma. Early reports estimated that patients with SS had up to 44 times increased risk of developing lymphoma compared with the general population. Chronic fatigue, depression, and a diminished quality of life are also common components of SS.
Classification
Two forms of the disease are recognized. Primary SS is the presence of sicca syndrome, xerostomia, or ‘dry mouth’, and xerophthalmia, or ‘dry eyes’ together, with no other autoimmune disease. Secondary SS is sicca syndrome plus another associated autoimmune disease such as rheumatoid arthritis (RA), SLE, or scleroderma. Based upon the classification criteria applied, the prevalence of SS may range from 0.5 to 3.0% of the population. All classification systems use a combination of both subjective and objective findings in the diagnostic process. The most recent 2002 criteria include subjective symptoms of dry mouth and dry eyes, and the following objective tests: ocular signs by Schirmer’s test and/or Rose Bengal score; focal sialadenitis by histopathology; salivary gland involvement by either salivary scintigraphy, parotid sialography or unstimulated salivary flow rate; and autoantibodies of SS-A/Ro and/or SS-B/La specificity. Box 3 presents the 2002 American-European Consensus Group Criteria for SS.
Etiopathogenesis
While a genetic predisposition to SS appears to exist, no simple Mendelian inheritance pattern has been demonstrated. Cases of two or more individuals with SS per family and SS in twins have been described. However, the level of genetic contribution is not known. Because large twin studies in SS are lacking, the twin concordance rate cannot be estimated. Familial clustering of different autoimmune diseases and co-association of multiple autoimmune diseases has been reported by Becker et al. Reports have also indicated that a SS proband may have relatives with other autoimmune diseases in approximately 30–35% of the cases. Assessing human leukocyte antigen (HLA)-DR and HLA-DQ gene segments in patients with SS reveals an increased use of haplotypes B, Drw52 and DR3. Correlations have been found between presence of HLA-DR haplotype and the presence of Ro/LA in SS. Gene polymorphisms have been analyzed, but no clear-cut relationship between these and primary SS have been identified. Clustering of non-major histocompatibility complex (MHC) susceptibility candidate loci in human autoimmune diseases supports a hypothesis that, in some cases, clinically distinct autoimmune diseases may be controlled by a common set of susceptibility genes.
Clinical features and diagnosis
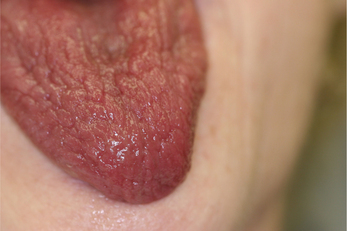
The most frequent oral signs and symptoms a dentist will encounter are xerostomia, a subjective sensation of oral dryness, and hyposalivation, or a diminished salivary flow rate. These will vary between patients, and the subjective dryness may not directly correlate with objective measures of hyposalivation. Initial indications of a diminished salivary output would be a lack of pooling in the floor of the mouth, thick or frothy saliva, and observing examination gloves sticking to the tongue or buccal mucosa. Patients may complain of difficulty chewing and swallowing, difficulty wearing their dentures, and altered taste. They will relate the necessity for drinking liquids to aid in swallowing food or to enhance their ability to speak. They may admit to keeping water by their bedside at night or frequently waking with a dry mouth. Patients with SS frequently carry water with them and often need to sip every 10–15 minutes during a consultation appointment. Upon intraoral examination, the tongue may appear fissured, slightly erythematous, and sometimes depapillated. If the patient complains of a ‘burning tongue’ and dysgeusia, oral candidiasis should be suspected (Figure 2). Salivary flow rate is diminished in primary SS patients compared with controls. In addition to quantitative changes, the protein content of the saliva is altered as well. Proteins necessary for buffering the oral acidity, countering fungal and microbial organisms may be altered. The lack of adequate salivary flow and qualitative changes in protein content may predispose the patient to dental decay, particularly in the cervical area, tooth loss, candidiasis and oral ulcerations (Figure 3). The change in saliva is linked to the lymphocytic infiltrate in the glands and subsequent damage to the functional units.
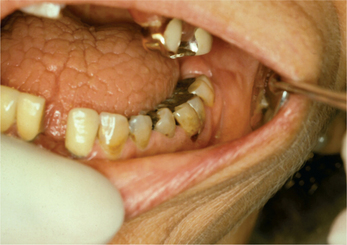
Figure 3 Oral cavity of Sjögren’s syndrome patient showing dental caries and fissured tongue. Courtesy: Dr Carol Stewart
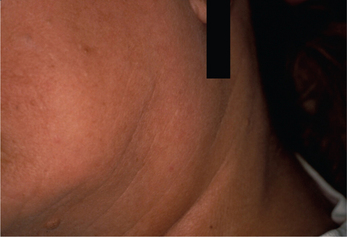
From one-fourth to two-thirds of patients with primary SS will have a diffuse enlargement of the major salivary glands during the course of their disease (Figure 4). This swelling may be unilateral or bilateral, intermittent or constant in nature. If the parotid swelling initially is unilateral, it often becomes bilateral with time. It commonly produces mild to no discomfort. However, diminished flow will enhance a patient’s susceptibility to bacterial infection in the glands and recurrent sialadenitis, which will produce pain. Although SS is considered primarily a disease of middle-aged females, it has also been reported in children and adolescents. Recurr ent bilateral parotitis in children and adolescents should include the possibility of SS in the differential diagnosis.
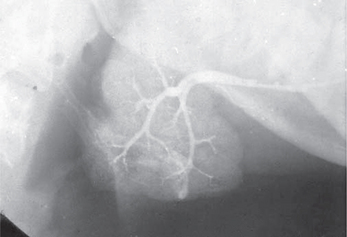
Conventional sialography renders useful information about the gland architecture and changes within it. Water-based radiopaque dye is injected into the major gland ducts followed by conventional imaging. Peripheral ducts within the glands are usually affected first with the inner ductal structure relatively well preserved. However, punctuate collections of the contrast material may be visible in the early stages of the disease followed by globular or larger collections as the condition progresses as shown in Figures 5–7. Once extensive intraglandular destruction has taken place and infection has become established, dilatation of central ducts is noted. Abscesses within the gland may be noted with a uniform distribution, unlike focal abscesses caused by other types of infections. However, sialography is an invasive procedure and other imaging modalities may be considered.
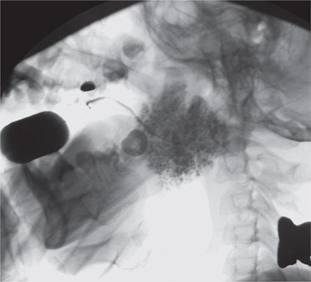
Figure 6 Sialograph of Sjögren’s syndrome of the submandibular salivary gland. Courtesy: Dr Ajit Auluck and Dr Chandrakant Shetty
Imaging with CT and MRI is common. The glands enlarge with time, assuming a denser appearance on CT. A honeycomb appearance is not infrequent but this is also seen with granulomatous conditions. Bilateral enlargement with cystic and solid lesions is noted (Figure 8A, B). In the early stage, the parotids appear normal. Multiple cysts appear during the intermediate stage. These eventually grow with time. If the mass assumes an invasive appearance, malignant transformation may be suspected. Foci of calcification within the glands are not uncommon. Heterogeneous enhancement may therefore be expected (Figure 9A, B). The glands could eventually appear smaller in size. In MRI, numerous punctuate areas appear within the glands with low signal intensity on T1 and T2 weighted images as the disease progresses. This is considered diagnostic of the condition. But, a lymphoma arising in a benign lymphoepithelial lesion (BLEL) does not have characteristic features that would help differentiate it from other tumors. However, it may be considered in patients who present with a parotid mass. Function of the glands is directly correlated with the amount of fat deposition, thereby indicating that a monitoring of this feature may be useful in diagnosing the condition. Another imaging technique that is useful is magnetic resonance sialography. It has been shown to be highly accurate with excellent sensitivity and specificity. Globular, punctuate or a lytic appearance is typical of the condition. If cysts develop within BLEL, these are detected using CT or MR. The absence of lymphadenopathy helps exclude HIV-related lymphoepithelial cyst formation.
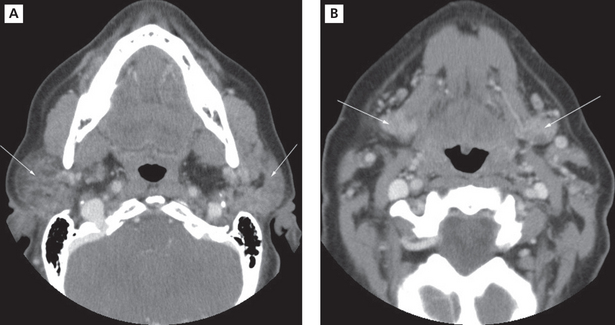
Figure 8 Sjögren’s syndrome. CT images showing diffuse sialadenitis affecting the submandibular and parotid glands bilaterally. Courtesy: Dr Madhu Nair
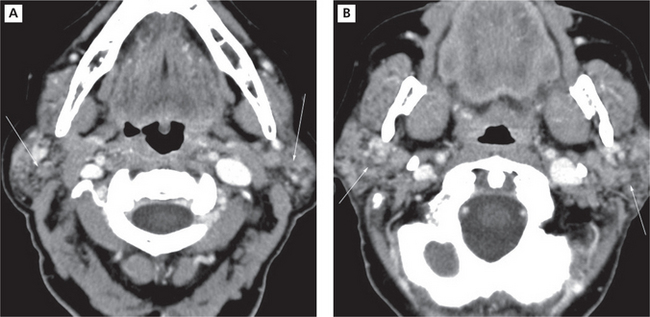
Figure 9 Chronic Sjögren’s syndrome. Contrast CT demonstrating a granular appearance of parotid glands that have been reduced in size Courtesy: Dr Madhu Nair
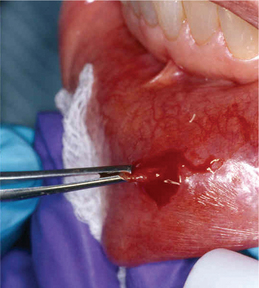
Lymphocytic infiltration in the lacrimal and salivary glands is a major feature of SS. One of the criteria for classification of SS includes the biopsy of the labial salivary glands. A 1.5-cm incision is made on normal appearing mucosa of the lower lip lateral to the midline (Figure 10). Five or more accessory salivary gland lobules are examined histopathologically for the presence of focal chronic inflammatory aggregates. A focus is 50 or more lymphocytes and some plasma cells within a 4 mm2 field in the salivary gland biopsy specimen. As noted in Figure 11A, B, these aggregates are adjacent to normal-appearing acini and are found throughout the glands. A finding of more than one focus within a 4 mm2 area of glandular tissue is supportive of a SS diagnosis. Manthorpe et al have reported an interesting fact that the focus scores are lower in SS patients who are cigarette smokers.
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses