Restorative Materials—Metals
Amalgam
An amalgam is an alloy of mercury and one or more other metals. Dental amalgam is produced by mixing liquid mercury with solid particles of an alloy containing predominantly silver, tin, and copper. Zinc and palladium may also be present in small amounts. This combination of solid metals is known as amalgam alloy. It is important to differentiate between dental amalgam and the amalgam alloy that is commercially produced and marketed as small filings, spheroid particles, or a combination of these, suitable for mixing with liquid mercury to produce the dental amalgam.
Once amalgam alloy is freshly mixed with liquid mercury, it has the plasticity that permits it to be conveniently packed or condensed into a prepared tooth cavity. After condensing, the dental amalgam is carved to generate the required anatomical features and then hardens with time. Amalgam is used most commonly for direct, permanent, posterior restorations and for large foundation restorations, or cores, which are precursors to placing crowns. Dental amalgam restorations are reasonably easy to insert, are not overly technique sensitive, maintain anatomical form, have reasonably adequate resistance to fracture, prevent marginal leakage after a period of time in the mouth, can be used in stress-bearing areas, and have a relatively long service life.
The principal disadvantage of dental amalgam is that its silver color does not match tooth structure. In addition, amalgam restorations are somewhat brittle, are subject to corrosion and galvanic action, may demonstrate a degree of breakdown at the margins of tooth and amalgam, and do not help retain weakened tooth structure. Finally, there are regulatory concerns about amalgam being disposed of in the wastewater. Despite these shortcomings, dental amalgam is a highly successful restorative material and is cost effective. However, alternatives such as cast gold, ceramics, and resin composite restorative materials are now competitive in terms of frequency of use. Many argue, however, that the use of amalgam must be strongly supported given its large public health benefit in the United States and many other countries.
In this chapter, the composition and morphology of the different dental amalgams are presented, followed by a discussion of low- and high-copper amalgams, the chemical reactions that occur during amalgamation, and the resultant microstructures. Various physical and mechanical properties are covered in the next section, as well as factors related to the manipulation of amalgam.
Dental Amalgam Alloys
History
Amalgam has been used for many years as a dental restorative material. Prior to 1900 many compositions were tried but few were successful when placed in the oral environment. Around 1900 scientific testing was applied to the problem, which led to a so-called balanced composition of silver and tin in the form of Ag3Sn with small amounts of copper and occasionally zinc added. Amalgam restorations made from this balanced formula were reasonably successful, and it was not uncommon to see 10- to 20-year-old amalgam restorations still in service. However, one disadvantage that remained was a propensity for fracture at the edge or margin of the amalgam restoration next to tooth structure, commonly called marginal fracture. Such fractures were considered to be exacerbated by the presence of the tin-mercury phase (Sn7-8Hg), which results when Ag3Sn reacts with mercury. This phase has been shown to be the weakest phase in the hardened amalgam and is subject to corrosive breakdown, particularly at the restoration/tooth margin where a most active form of corrosion known as crevice corrosion is likely to occur. Although a significant amount of marginal fracture was exhibited among the commercial alloys at the time, this type of fracture was accepted as an indigenous characteristic of dental amalgam restorations.
In 1962 a new amalgam alloy, called Dispersalloy, was introduced that consisted of the addition of a spherical silver-copper eutectic particle to the traditional lathe-cut Ag3Sn particle in a ratio of 1:2. The mixing of these two types of particles led to the term admix alloy. Although the rationale for this admix alloy was to strengthen the amalgam, an unanticipated but highly significant benefit proved to be the elimination of the tin-mercury phase (Sn7-8Hg). This elimination occurred because the increased copper in the silver-copper eutectic reacted preferentially with tin so that Sn7-8Hg could not form. Early results from the clinical use of this new amalgam showed what appeared to be improved marginal integrity. However, it took carefully designed clinical studies to show, unequivocally, that the extent of marginal fracture was either eliminated or reduced significantly in restorations made from Dispersalloy.
About 10 years later, another alloy, called Tytin, was introduced using the same principle of adding increased copper to eliminate the tin-mercury phase. However, in this case, this was done by combining a significant amount of Cu3Sn together with Ag3Sn in the form of a unicompositional spherical particle.
Both of these relatively new alloys raised the copper content from 5%, present in the older balanced composition alloy, to about 13% for the newer alloys. Thus, the term low-copper alloys refers to alloys that do not contain sufficient copper to prevent the formation of Sn7-8Hg, whereas the term high-copper alloys refers to alloys that do have sufficient copper to prevent the formation of Sn7-8Hg.
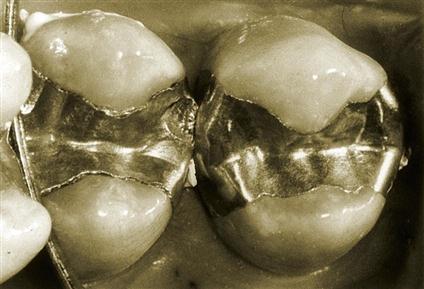
In Figure 10-1, two restorations are shown, after 3 years of clinical service, that were placed at the same time in the same patient. The restoration on the left was made from a low-copper alloy, whereas the restoration on the right was made from a high-copper alloy. The higher extent of marginal fracture for the low-copper alloy is clearly shown.
Notice also that in the low-copper restoration, deep indentations were produced by occlusal forces acting through the cusps of opposing teeth that is not shown in the high-copper restoration. This observation suggests that the viscoelastic behavior or creep of dental amalgam has clinical implications.
Composition and Morphology
ANSI/ADA (American National Standards Institute/American Dental Association) specification No. 1 (ISO 24234) for amalgam alloy includes a requirement for composition. This specification does not state precisely what the composition of alloys shall be; rather, it permits some variation in composition. Specifically, it states that the chemical composition shall consist primarily of silver, tin, and copper. Indium, palladium, platinum, zinc, or mercury may also be included in lesser amounts.
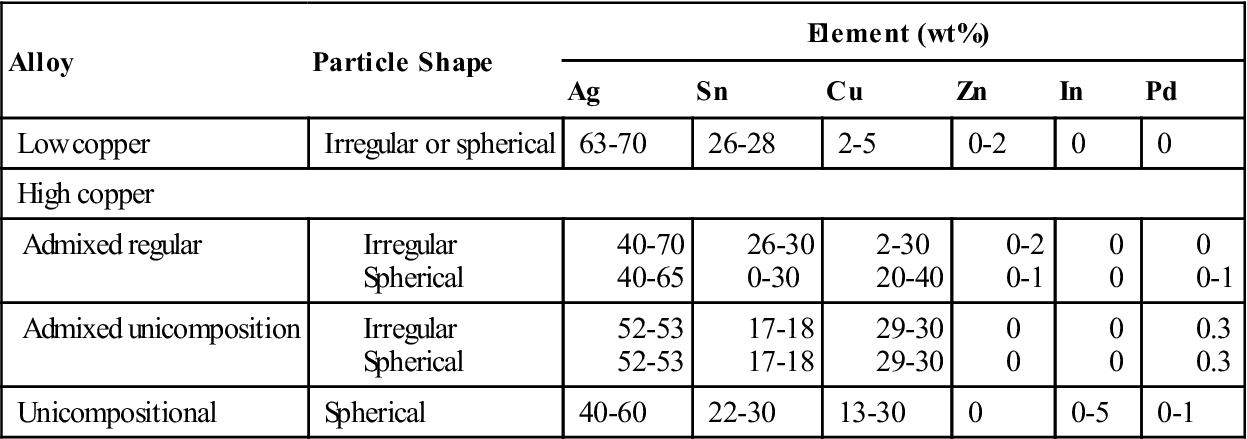
The ANSI/ADA specification also includes a notation about the presence of zinc in amalgam alloys, with more than 0.01% zinc classified as zinc containing and those with less than 0.01% as nonzinc alloys. Zinc has been included in amalgam alloys as an aid in manufacturing; it helps produce clean, sound castings of the ingots used for producing cut-particle alloys. However, the presence of zinc has been shown to produce a delayed expansion if water-based fluids such as blood or saliva are present within the amalgam during condensation. Therefore, practitioners are informed of this possible problem. Possible remedies are the use of zinc-free alloys and careful attention given to rubber dam isolation of the prepared tooth. The presence of contamination within the amalgam must be avoided because it can degrade the integrity of the restoration. The approximate composition of commercial amalgam alloys are shown in Table 10-1.
TABLE 10.1
Approximate Composition of Low- and High-Copper Amalgam Alloys
< ?comst?>
< ?comen?>< ?comst1?>
< ?comst1?>
< ?comen1?>
Ag, Silver; Cu, copper; In, indium; Pd, palladium; Sn, tin; Zn, zinc.
The alloys are broadly classified as low-copper (5% or less copper) and high-copper alloys (13% to 30% copper). Particles are irregularly shaped microspheres of various sizes or a combination of the two. Scanning electron micrographs of the particles are shown in Figure 10-2. The low-copper alloys have either irregular or spherical particles. Both morphologic types contain silver and tin in a ratio approximating the intermetallic compound Ag3Sn. High-copper alloys contain either spherical particles of the same composition (unicompositional) or a mixture of irregular and spherical particles of different or the same composition (admixed).
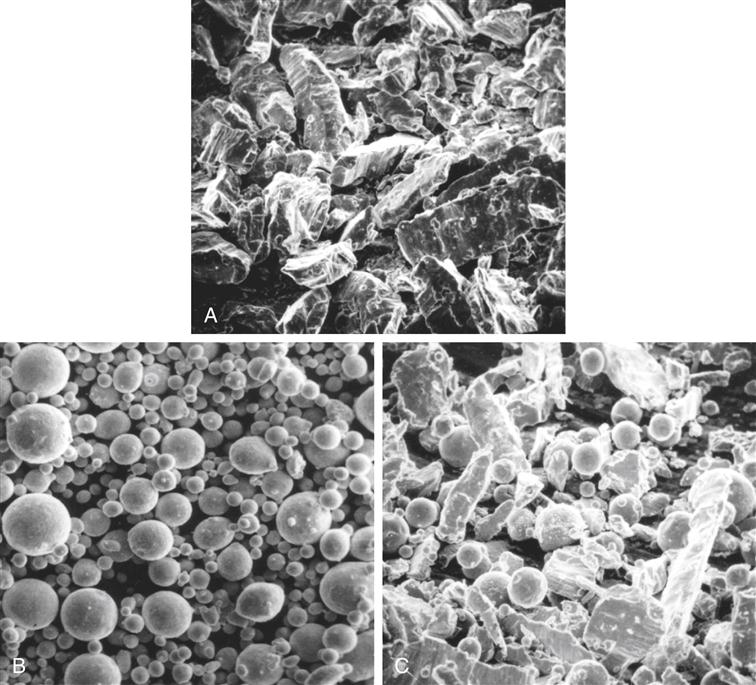
A, Lathe-cut; B, spherical; and C, admixed amalgam alloys.
When the particles have different compositions, the admixed alloys are made by mixing particles of silver and tin with particles of silver and copper. The silver-tin particle is usually irregular in shape, whereas the silver-copper particle is usually spherical. The composition of the silver-tin particles in most commercial alloys is the same as that of the low-copper alloys. Different manufacturers, however, have somewhat different compositions for the silver-copper particle. The compositional ranges of the spherical silver-copper particles are shown in Table 10-1. The admixed regular alloy contains 33% to 60% spherical particles that have a composition close to the eutectic composition of Ag3Cu2 (see Figure 10-2); the balance is irregular particles.
Like the admixed alloy, the unicompositional alloys have higher copper contents than the traditional lathe-cut or spherical low-copper alloys, but all the particles are spherical, as seen in Figure 10-2. The silver content of the unicompositional alloys varies from 40% to 60%, copper content varies from 13% to 30%, and tin content varies only slightly.
A high-copper admixed alloy is also available, in which both spherical and irregular particles have the same composition and the copper content is between 29% and 30%. High-copper alloys are less commonly supplied as unicompositional and irregular particles. The lathe-cut, high-copper alloys contain more than 23% copper.
It is estimated that more than 90% of the dental amalgams currently placed are high-copper alloys. Of the high-copper alloys, admixed alloys are used more often than spherical types, and fewer irregularly shaped or lathe-cut types are selected. A high-copper alloy is selected to obtain a restoration with high early strength, low creep, good corrosion resistance, and good resistance to marginal fracture.
In general, alloy composition—particle size, shape, and distribution—and heat treatment control the characteristic properties of the amalgam.
Production
Irregular Particles
To produce lathe-cut alloys, the metal ingredients are heated and protected from oxidation until melted, then poured into a mold to form an ingot. The ingot is cooled relatively slowly, leading to the formation of mainly Ag3Sn (γ) and some Cu3Sn (ε), Cu6Sn5 (η′), and Ag4Sn (β). After the ingot is completely cooled, it is heated for various periods of time (often 6 to 8 hours) at 400° C to produce a more homogeneous distribution of Ag3Sn. The ingot is then reduced to filings by being cut on a lathe and ball milled. The particles are passed through a fine sieve and then ball milled to form the proper particle size. The particles are typically 60 to 120 μm in length, 10 to 70 μm in width, and 10 to 35 μm in thickness. Most products are labeled as fine-cut. The particle size and shape of lathe-cut amalgam alloys are shown in Figure 10-2, A.
In general, freshly cut alloys amalgamate and set more promptly than aged particles, but some aging of the alloy is desirable to improve the shelf life of the product. The aging is related to relief of stress in the particles produced during the cutting of the ingot. The alloy particles are aged by subjecting them to a controlled temperature of 60° to 100° C for 1 to 6 hours. Irregularly shaped high-copper particles are made by spraying the molten alloy into water under high pressure.
Spherical Particles
Spherical particles of low- or high-copper alloys are produced when all the desired elements are melted together. In the molten stage the metallic ingredients form the desired alloy. The liquid alloy is then sprayed, under high pressure of an inert gas, through a fine crack in a crucible into a large chamber. Depending on the difference in surface energy of the molten alloy and the gas used in the spraying process, the shape of the sprayed particles may be spherical or somewhat irregular, as shown in Figure 10-2, B. The diameter of the spheres varies from 2 to 43 μm.
Silver-Tin Alloy
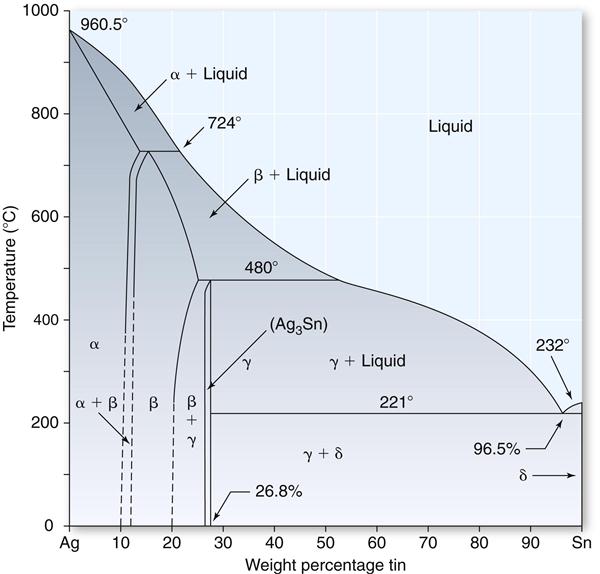
Because two of the principal ingredients in the amalgam alloy are silver and tin, it is appropriate to consider the equilibrium phase diagram for these two metals, as shown in Figure 10-3.
Ag, Silver; Sn, tin. (Modified from Murphy AJ, Inst. Metals. J. 35, 107, 1926.)
The most important feature in this diagram concerning the silver-tin alloy is that when an alloy containing approximately 27% tin is slowly cooled below a temperature of 480° C, an intermetallic compound (Ag3Sn) known also as the gamma (γ) phase is produced. This Ag3Sn compound is an essential ingredient in the silver amalgam alloy and combines with mercury to produce a dental amalgam of desired mechanical properties and handling characteristics. This silver-tin compound is formed over only a narrow composition range. The silver content for such an alloy would be approximately 73%. Practically, the tin content is held between 26% and 30%, and the remainder of the alloy consists of copper, and in some cases, zinc. If the concentration of tin is less than 26%, the beta (β) phase, which is a solid solution of silver and tin, forms. The replacement of silver by an equal amount of copper produces a copper-tin compound (Cu3Sn).
In general, larger (greater than 30%) or smaller (less than 26%) quantities of tin in the alloy are detrimental to the final properties of the amalgam. The reason for this unfavorable shift in properties is generally considered to be a reduction in the amount of Ag3Sn as the percentage of tin is altered beyond the indicated limits. This is the basis for the rather narrow limits of the alloy compositions of current products with acceptable properties.
Silver-tin amalgam alloys compounded to produce largely Ag3Sn react favorably with mercury to produce only slight dimensional setting changes when properly manipulated. In addition, setting time is shortened by increasing silver content, and mechanical properties are superior when an alloy of Ag3Sn is used rather than one with higher tin content.
Amalgamation Processes
Low-Copper Alloys
All dental amalgam alloys, including both low- and high-copper types, have Ag3Sn as their primary component, which reacts with mercury to form Ag2Hg3, the major matrix phase of the set amalgam. Because of this basic similarity, an initial discussion of the amalgamation of the low-copper alloys is a significant preliminary to that of the high-copper alloys.
The amalgam alloy is intimately mixed with liquid mercury to wet the surface of the particles so that the reaction between liquid mercury and alloy can proceed at a reasonable rate. This mixing is called trituration, which has the dual function of mixing the ingredients and removing surface oxide layers that have formed on the alloy particles. During this process, mercury diffuses into the alloy particles and reacts with the silver and tin portions of the particles to form predominantly silver-mercury and tin-mercury compounds. The silver-mercury compound is Ag2Hg3 and is known as the gamma one (γ1) phase, and the tin-mercury compound is Sn7–8Hg, known as the gamma two (γ2) phase. However, the silver-tin, silver-mercury, and tin-mercury phases are not pure. For example, the alloy particles usually contain small amounts of copper in the form of Cu3Sn and occasionally small amounts of zinc. Ag2Hg3 contains 1% to 2% of tin.
While crystals of the γ1 and γ2 phases are being formed, the amalgam is relatively soft and easily condensable and carvable. As time progresses, more crystals of γ1 and γ2 are formed; the amalgam becomes harder and stronger and is no longer condensable or carvable. The lapse of time between the end of the trituration and when the amalgam hardens and is no longer workable is called working time.
The amount of liquid mercury used to amalgamate the alloy particles is not sufficient to react with the particles completely. Therefore, the set mass of amalgam contains about 27% unreacted particles. A simplified reaction of a low-copper amalgam alloy with mercury can be summarized in the following manner:
< ?xml:namespace prefix = "mml" ns = "http://www.w3.org/1998/Math/MathML" />
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses