Chapter 10
Radiopacities
Introduction
A radiopacity is the “white” area on a conventional radiograph. it represents a tissue or a structure within the patient, which attenuates the primary beam of X-rays more than adjacent tissue or structures. In the normal patient presenting to the oral and maxillofacial practitioner, the normal radiopaque structures are anatomical: the teeth, the bones of the jaws (including the middle-third of the face and nasal bones), the stylohyoid complex (including the hyoid bone), the skull base, and cervical vertebrae. Although the term radiopacity can be used for any such tissue or structure it is frequently applied only to those, which suggest a lesion or disease process. These radiopacities are due to deposition of mineralized tissue. This deposition reflects two different processes. Deposition either by bone cells in dysplastic and neoplastic lesions or by nonbone cells in dystrophic processes. The last arise usually in chronic inflammatory lesions or those with multiple episodes of inflammation. Dystrophic calcification is commonly observed in the soft tissues of the jaws as calcification of the tonsils (now usually secondary to tonsillitis) or cervical lymph nodes. It also occasionally presents in atherosclerotic plaques in blood vessel walls.
In addition to conventional radiography, advanced imaging modalities such as computed tomography (CT) and magnetic resonance (MRI) are frequently used to investigate jaw lesions. Although calcified lesions and structures are still white on images made by either helical computed tomography (HCT, see Chapter 4) or cone-beam computed tomography (CBCT, see Chapter 5), they appear black on MRI as do the air-filled spaces and blood vessels (see Chapter 6).
The term significant will be used only when the feature it is qualifying is P < 0.05. Table 10.1 overviews the causes of radiopacities of the jaws. Because almost all of these have been addressed in other texts, they will not be pursued further here. For radiopacities within the bony jaws, refer to Figures 10.1 and 10.2.
Table 10.1. Radiopacities of the jaws
| Step | Cause of Radiopacity |
| 1. | Development Artifact |
| “White metal-like spots” | |
| Fixer on undeveloped film | |
| 2. | Inadequate Patient Preparation |
| Jewelry (earrings, necklace, facial, and tongue piercing) | |
| Hair ornaments | |
| Hearing aid | |
| Removable dentures and orthodontic appliances | |
| 3. | Normal Anatomy and Variants |
| Radiopacity over roots of mandibular premolars (on panoramic radiograph), perhaps bilateral | |
| Torus mandibularis | |
| Linear or ovoid opacities in near vertical axis behind or superimposed on angle of mandible | |
| Stylohoid complex | |
| A vertical fingerlike radiopacity usually with a reticular or honeycomb pattern below greater horn of the hyoid | |
| Thyroid; superior horn | |
| 4. | Metal-like/Iatrogenic |
| Restorative material | |
| Overextended root filling | |
| Amalgam in socket | |
| Amalgam retrograde with apicectomy | |
| Broken instrument | |
| Endodontic | |
| Elevator | |
| Surgical packs. | |
| Bone plates/wires | |
| Implants | |
| 5. | Radiopacity/ies in the soft tissues |
| Round | |
| Neck; in vertical line (dystrophic from behind ramus to thoracic inlet | |
| Lymph nodes | |
| a. TB in the elderly or Indian subcontinent patients | |
| b. Sarcoidosis | |
| c. Previous radiotherapy treated lymphoma | |
| Neck; broadly in horizontal line from: | |
| a. Behind ramus to 2nd maxillary molar | |
| Parotid sialoliths | |
| b. Below gonial notch | |
| Submandibular sialoliths | |
| Group centered about mandibular foramen | |
| Tonsilloliths | |
| Single, anywhere | |
| Acne scar (obvious skin scar) | |
| Multiple, anywhere | |
| Gardner’s Syndrome | |
| Target and round; anywhere with perhaps discoloration of overlying skin | |
| Hemangioma | |
| Sheets | |
| a. Generalized | |
| Scleroderma | |
| b. Localized | |
| Myositis ossificans | |
| Bars | |
| Cystercicosis (parasite) | |
| Parallel lines | |
| Arteriosclerotic blood vessels (diabetes OR chronic renal disease) | |
| 6. | Radiopacities within the Bony Jaws |
Figure 10.1. Radiopacities occurring within the bony jaws.
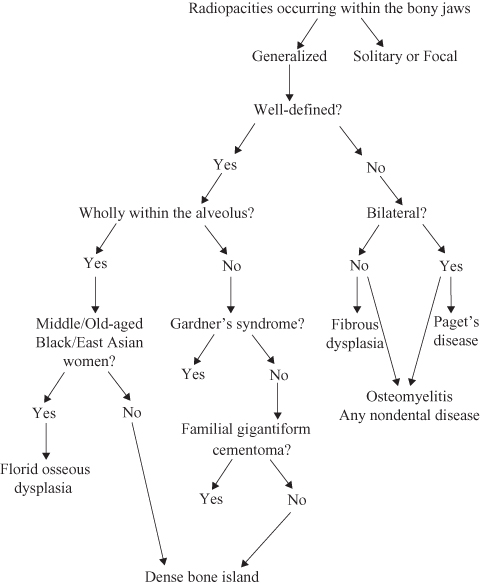
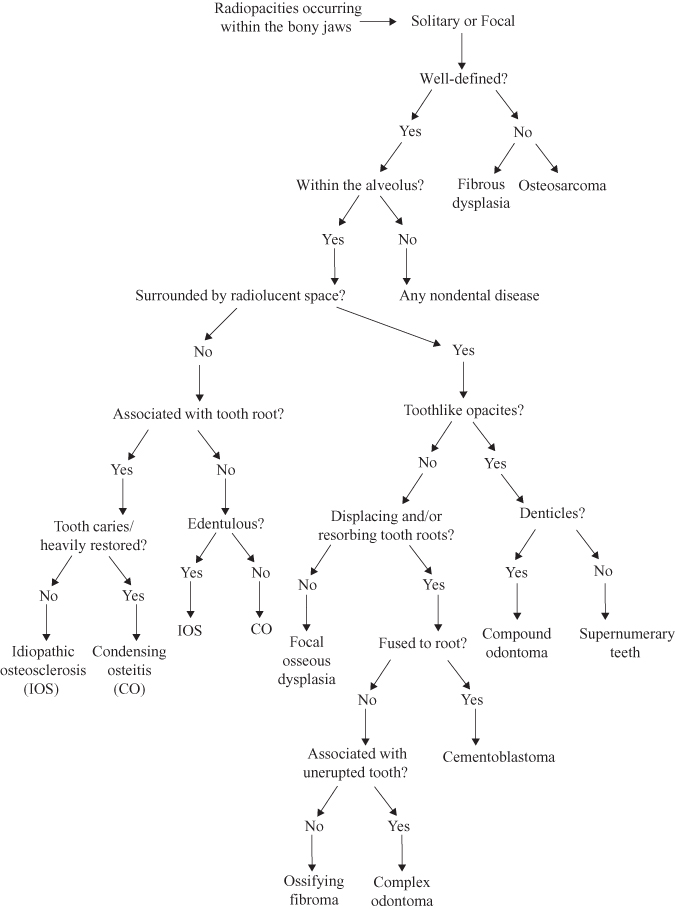
Figure 10.2. Radiopacities occurring within the bony jaws: solitary or focal.
Radiopacities Outside the Bony Jaws
CALCIFICATIONS OF THE STYLOHYOID COMPLEX
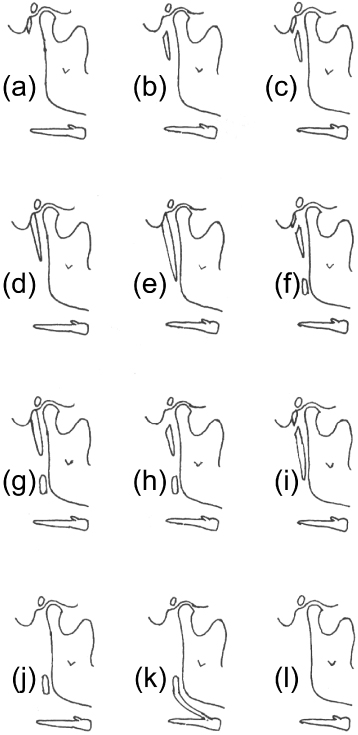
The 12 most common patterns of calcification of the stylohyoid complex are set out in Figure 10.3.1 These are based on the four developmental regions of this complex: the tympanohyal (skull base), stylohyal (majority of the styloid process), ceratohyal (contributes to elongated styloid process and stylohyoid ligament), and hypohyal (lesser horn of hyoid component of stylohyoid ligament). This system of patterns was introduced for clinical use because traditional measurement from panoramic radiographs is subject to substantial magnification and distortion, especially in the region posterior to the alveolar processes.1
Figure 10.3. The 12 patterns of calcification of stylohyoid complex used by MacDonald-Jankowski (2001). Pattern: (a) Region 1 = tympanohyal alone; (b) Region 2 = stylohyal alone; (c) Region 1 and 2, separate; (d) Regions 1 and 2 continuous; (e) Regions 1, 2, and 3 continuous; (f) Regions 1, 2, and 3 separate; (g) Regions 1 and 2 continuous, but separate from 3; (h) Regions 2 and 3 separate; (i) Regions 2 and 3 continuous, but separate from 1; (j) Region 3 alone; (k) Region 3 and 4 continuous (may include calcification in one other region); (l) No styloid process visible. Note 1: Regions 1, 2, 3, and 4 coincide with the 4 centers of ossification of the stylohyoid complex, tympanohyal, stylohyal, ceratohyal, and hypohyal. Note 2: Patterns (a) to (d) are normal styloid processes, pattern (e) is an elongated styloid process, and patterns (f) to (k) are calcified stylohyoid ligaments.
The following paragraphs summarize the different prevalences of the more common patterns in two world communities, Hong Kong and London. The following percentages are cited in that order. A normal styloid process (patterns a to d; 84% to 73%) and pattern d on its own (67% to 40%) were significantly more prevalent in the Hong Kong Chinese (Figures 10.4, 10.5), whereas a calcified stylohoid ligament (patterns f to k) were significantly more frequent in Londoners (4% to 16%) (Figure 10.6). Segmentation was significantly more frequent in Londoners (6% to 23%). Bilateral symmetry, with regard to pattern, was significantly more frequent in the Chinese (100% to 93%) (Figures 10.5, 10.7). There was no significant difference between Hong Kong and London for an elongated styloid process (pattern e; 9% and 8%, respectively) (Figure 10.7).
Figure 10.4. Panoramic radiograph displaying a normal styloid process. A normal styloid process can extend as inferior as the middle of the mandibular foramen. This example is of a continuous bone and is comprised of the tympanohyal and stylohyal components (pattern [d]).
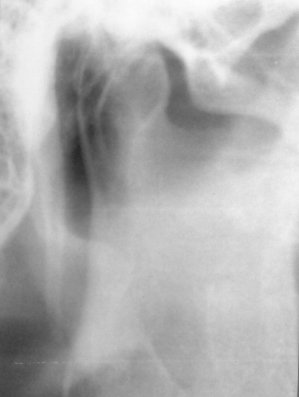
Figure 10.5. Coronal computed tomography (CT) displays the styloid process within the bone (a) and soft-tissue (b) windows. The coronal section is at the level of the anterior arch of C1. The styloid processes on these coronal sections are angled medially, running almost straight (in part tracing out the stylohyoid ligament) toward the junction between the body and the greater horn of the hyoid bone. The greater horn on each side are observed in cross-section.
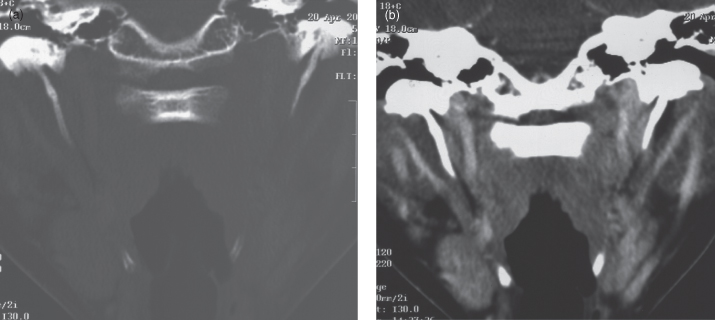
Figure 10.6. Panoramic radiograph displayed a bilateral and symmetrical calcified stylohyoid complex.
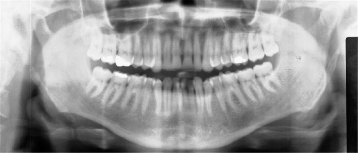
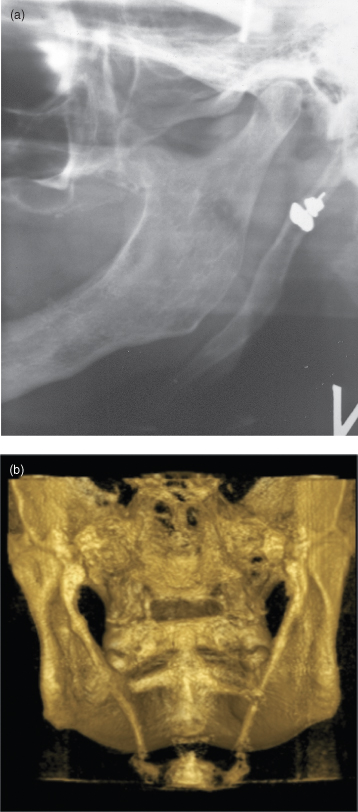
Figure 10.7. The anterior (a) and medial (a) path of the calcified stylohyoid ligament. (a) Panoramic radiograph showing a long styloid process extending inferiorly to as far as the hyoid bone. A long styloid process includes the tympanohyal and stylohyal components of the normal styloid process, but also includes the ceratohyal component. (b) Cone-beam computed tomography (CBCT) of a completely calcified stylohyoid ligament.
Figure courtesy of Dr. Alexandre Khairallah, University of Lebanon.
Using the same table, Okabe et al.2 observed similarity in pattern distribution between their 80-year-old Japanese and the above Hong Kong Chinese. They suggested that this was perhaps due to similarities in East Asian genotypes and phenotypes. The major difference was that their sample displayed a substantially greater proportion of pattern e (35%). As the Okabe et al.2 study was exclusively 80-year-olds, this difference was considered to be a phenomenon of aging. This was supported by a Brazilian report measuring directly from panoramic radiographs.3 Okabe et al.2 also reported that the elongated styloid process (Figure 10.7) correlated significantly with increased serum calcium concentration and heel bone density. It also correlated significantly with the patient’s height and weight. Therefore, these findings “may provide potentially life-saving information about elderly people.”2
The styloid process also runs in an anterior and medial direction to the hyoid bone (Figures 10.7a and b, respectively); the inferior horn and upper half of the body of the hyoid bone form the caudal component of the stylohyoid complex.
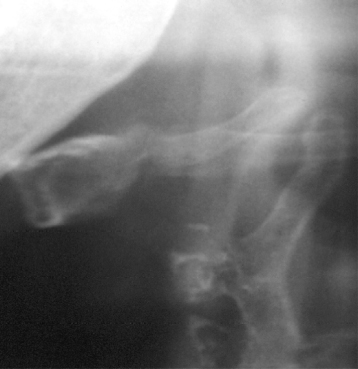
The upper half of the hyoid bone was derived in common with the cranial components of the stylohyoid complex from the second pharyngeal arch or Reichert’s cartilage. The rest of the hyoid is derived from the third pharyngeal arch. The hyoid bone arises from 6 centers of ossification, 2 for the body and 1 for each horn.4 The lesser horn may not fuse with the body (see Figure 1.13b), but it is attached by fibrous tissue to the greater horn, which in turn articulates with the body by a diarthrodial synovial joint (Figure 10.8). This last feature is important, because it is clearly observed on panoramic and lateral cephalometric radiographs, particularly in children. It should not be mistaken for a hyoid bone fracture.5
Figure 10.8. Panoramic radiograph displays the hyoid bone immediately below the angle of the mandible. Below the hyoid bone is the superior horn and partially calcified lateral lamina of the thyroid cartilage. In reality, in contrast to its traditional display in many anatomical texts, the hyoid bone infrequently exists as a complete unified bone, except in the oldest patients. The various parts are more frequently observed radiologically as separate bones joined by radiolucent spaces, representing the diarthrodial synovial joints. In this figure the anterior rectangular bone represents the body of the hyoid, whereas the small round bone superimposed and or in contact with its superiodistal margin is the lesser horn. Immediately distal to the above joint is a horizontal V whose upper limb represents the image of the ipsilateral greater horn. The lower, longer, almost horizontal image represents the contralateral greater horn. Calcification of the thyroid cartilage is encountered increasingly in the older patient. Nevertheless, as is obvious here, it can coexist with a hyoid bone displaying all of its components as still separate bony entities.
The stylohyoid complex infrequently causes difficulty in its recognition, except perhaps pattern j in which no other landmarks of the stylohyoid complex are available. This could be mistaken for calcified carotid artery atheroma. Occasionally, a long styloid process may cause Eagle’s syndrome, which among other features may cause atypical pain.6
CALCIFIED CAROTID ARTERY ATHEROMA
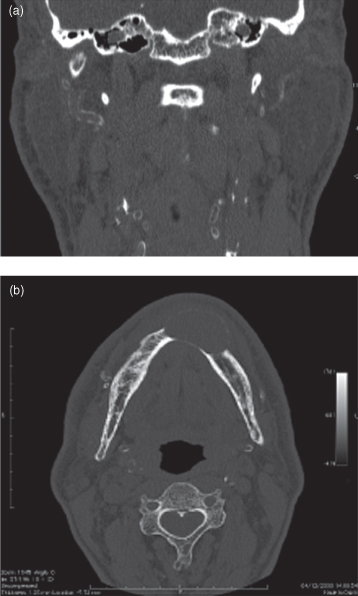
There are two types of calcification of the arteries depending upon whether the tunica media or tunica intima is involved. The former is termed medial calcific sclerosis or Monckeberg’s arteriosclerosis, whereas the latter, because of its narrowing of the lumen and atheroma formation is termed calcified carotid artery atheroma (CCAA) (Figure 10.9a). The former’s synonym, Monckeberg’s arteriosclerosis, implies vessel hardening and may be radiologically apparent as a tramline or pipestem pattern on conventional radiography and computed tomographic sections (Figure 10.9). Although supposedly benign in comparison to CCAA in outcome, Monckeberg’s arteriosclerosis can be associated with medical conditions such as parathyroidism and osteoporosis. Most commonly observed in the limbs, it infrequently presents in the head and neck. Although the definitive diagnosis for calcification of the tunica intima or tunica media rests on histopathology, biopsy of an artery has its own obvious risks.
Figure 10.9. Computed tomography (bone windows and no contrast media used) of parathyroidism secondary or tertiary to chronic kidney disease displaying calcification. Monckeberg’s arteriosclerosis of the branches of the external carotid artery. The classical stempipe or railtrack pattern of calcification of the arteries tunica media is evident. (a) A coronal section (bone window), at the level of the anterior arch of the first cervical vertebra, displays the rostral part of the external carotid artery. (b) An axial section (bone window), at the level of the second cervical vertebra, displays the “brown tumor,” as a expansile unilocular radiolucency, which was considered on clinical and conventional radiological examination to be an ameloblastoma. Monckeberg’s arteriosclerosis plots the torturous course of the facial artery medial and lateral to the mandible. There is a short arc of calcification within the internal carotid artery.
Figures courtesy of Dr. Lewei Zhang, Oral and Maxillofacial Pathology, Faculty of Dentistry, UBC.
CCAA presents as a round radiopacity initially and becomes linear as it becomes larger; it frequently presents as 2 parallel vertical lines. It is sited at or below the intervertebral space between the third and fourth cervical vertebrae.7 The CCAA observed on panoramic radiographs was first reported by Friedlander and Landes in 1981.8 Since then many reports have indicated that this phenomenon is widespread, affecting almost every global group (see Chapter 1 for their definitions). So far there does not appear to be a report from the sub-Saharan Africa. Nevertheless, the clinical significance of the CCAA and the utility of the panoramic radiograph as a screening tool for the CCAA are very controversial. Their “results suggest that the presence of carotid artery calcifications on panoramic radiographs may be related to the history of past vascular diseases; however, this is not a useful marker for subsequent vascular diseases and related death among 80-year-olds.”9 This was supported by a systematic review, which concluded that clinical guidelines based upon the hypothesis that CCAA, detectable on panoramic radiographs, is associated with an increased risk of stroke … cannot be established on the basis of the current evidence.”10 Furthermore, Madden et al.11 reported that panoramic radiography, when compared to ultrasonography, is not a reliable means to detect CCAA or stenosis. This poor assessment of the panoramic radiograph was qualified by Damaskos et al.12 Furthermore, Friedlander and Cohen maintain that “incidental finding of a CCAA … portents significant risk of a future adverse vascular event.”13 They observed that 15% of such cases had occult metabolic disease, formerly known as insulin-resistant syndrome. This syndrome is composed of increased abdominal obesity, raised triglycerides, reduced high density lipids and cholesterol, hypertension, and insulin resistance. A referral in such cases is necessary “because aggressive management may preclude a stroke.”13 Finally, developing Maddens et al.’s conclusions, Farman states that patients for whom CCAA have been detected should be further screened by ultrasonography.14
TRITICEOUS CARTILAGE (TRITICAE CARTILAGO)
The thyroid cartilage complex can undergo calcification (Figures 10.8, 10.9a) with increasing age. Calcification of the thyroid cartilage itself is infrequently mistaken for CCAA. On the other hand, the triticeous cartilage is more frequently mistaken for CCAA, particularly by students. Their shape, outline, and location assist in distinguishing them. The triticeous cartilage lies within the lateral thyrohyoid ligament between the superior horn of the thyroid (if apparent) and the distal end of the greater horn of the hyoid. It is round and of homogeneous density, whereas the CCAA is round initially and becomes linear as it becomes larger, frequently as 2 parallel vertical lines. The triticeous cartilage is an intrinsic component of the larynx and serves as an attachment of the vocal cords and the musculature responsible for phonation. It undergoes calcification more frequently in females (12%) than in males (8%).7
TONSILLOLITHS
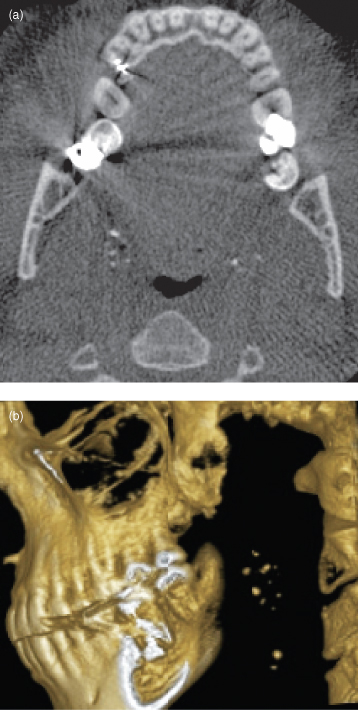
Tonsilloliths affecting the palatine tonsil are frequently observed on panoramic radiographs and are now becoming increasingly observed on CBCT (Figure 10.10). In the former they are most commonly observed superimposed upon the mandibular foramen. Formerly they were associated with tubercular lesions in the older patient, but are now observed in younger patients, presumably secondary to multiple episodes of tonsillitis earlier in life. These tonsilloliths may be associated with halitosis.15 At least one has caused dysphagia.16 They also present on CT17 and MRI.18
Figure 10.10. Cone-beam computed tomography (CBCT) of tonsilloliths. (a) Axial CBCT showing tonsilloliths adjacent to the oropharynx just medial to the mandibular foramen. (b) Three-dimensional reconstruction, cutting away the posterior body and vertical ramus of the mandible to display the tonsilloliths.
Figures courtesy of Dr. Alexandre Khairallah, University of Lebanon.
OTHER NONPATHOLOGICAL CAUSES OF RADIOPACITIES
Other structures that can appear as radiopacities, but are not included in Table 10.1, are ironically soft-tissue structures. These include not only normal anatomical structures such as the tongue, the soft palate, and the pharynx clearly apparent on the panoramic radiograph, but they also include soft-tissue lesions within the maxillary antrum. All these appear radiopaque by virtue of being contrasted (silhouetted) against the air-filled space. The reason for this phenomenon is discussed in Chapter 11.
Artifacts causing radiopacities arise from three main sources: image development, inadequate preparation, and earlier treatment (Table 10.1). Although image development artifacts in the traditional chemistry-based technology (film) and the strategies to avoid them are well known, the recent advent of the digital imaging technologies have the potential for different artifacts. The photostimulable phospher plates are easy to damage, resulting in white scratches and bite marks.19
Although artifacts caused by metal restorations (amalgam restorations, crowns, and bridges) in conventional imaging are extremely infrequent, even in panoramic radiography with its secondary imaging of the contralateral jaws (except for long or large earrings), this is not true for advanced imaging. In spite of the development of metal artifact reduction (MAR) software metal dental restorations pose significant problems for HCT (see Figure 4.9), CBCT (see Figures 5.2a, 5.4), and MRI (see Figure 6.6).
Radiopacities Occurring within the Bony Jaws
The flowcharts in Figures 10.1 and 10.2 generally flow from the most important clinical and radiological findings, addressing systemic lesions and malignancies first. Multiple radiopacities, particularly if they are distributed throughout the jaws, suggest a systemic cause, whereas the single radiopacity suggests a local cause.
The degree of marginal definition is crucially important to determining potentially serious disease. If it is well defined, the radiopacity is more likely to be benign, whereas a poorly defined radiopacity, in addition to inflammation or fibrous dysplasia, could represent a malignancy. The radiopacity’s relationship to the mandibular canal or the image of the hard palate (on panoramic or cephalometric radiographs) indicates whether it is likely to be of odontogenic origin. A radiopacity occurring above the mandibular canal or below the image of the hard palate is within the dental alveolus and therefore could be of odontogenic origin.
If the radiopacity is sited within the alveolus, its relationship to teeth is important, in order to refine further the differential diagnosis. If it is associated with the root of an erupted tooth, which has a large carious lesion or a large restoration, suggesting the possibility of a necrotic pulp, inflammation is a likely cause. If the radiopacity is associated with the crown of an unerupted tooth, an odontogenic lesion, most likely a neoplasm, should be considered.
The effect of the radiopacity on the tooth or adjacent structures is manifested by either displacement or erosion. The latter when applied to teeth, particularly their roots, is termed root resorption. Although all lesions presenting as radiopacities may in due course cause root resorption, this would appear to be a particular feature of certain odontogenic neoplasms. Displacement of teeth and buccolingual cortices are universal to all expansile lesions.
The flowcharts focus on the most common and important lesions and are not exhaustive with regard to the rarer lesions, particularly if they respond well to the initial treatment—i.e., they are very unlikely to recur.
Multiple Radiopacities
Multiple or widely distributed lesions suggest a systemic rather than a local cause. Therefore, it is necessary to identify such lesions early. Although these lesions are not common, failure to identify them early may have significant implications for the patient’s continued well-being. Polyostotic fibrous dysplasia, particularly the McCune-Albright syndrome, will have already been diagnosed early in life. Paget’s disease and Gardner’s syndrome are very important lesions that present later in life. Before proceeding to these lesions, a unique radiological phenomenon should be introduced: leontiasis ossea.
LEONTIASIS OSSEA
Leontiasis ossea, although infrequently observed, is important because it represents important lesions such as Paget’s disease of bone and hyperparathyroidism. Its name precisely reflects an appearance of a lion’s maxilla. Classically, both cheeks are very full and the external nose is small. This last effect is achieved by the following: the external nose, which itself remains essentially unchanged, becomes submerged within the outwardly expanded anterior wall of the maxilla. As a result the external nose is now both relatively smaller and flatter due to a more obtuse angle formed between the alae.
The presentation on CT of the internal structure is usually ground glass or an extensive network of serpentine channels within the radiopacity. Its frequently bilateral presentation usually distinguishes it from fibrous dysplasia.
PAGET’S DISEASE OF BONE
Paget’s disease of bone (PDB) was originally called “osteitis deformans” by Paget himself, which vividly describes this disease. It is characterized by rapid bone remodeling and the deposition of structurally abnormal bone.20 Although it classically affects individuals older than 40 year of age, a subset of patients are juveniles. The last is called “early-onset familial Paget’s disease of bone” and is primarily genetic.21 Although its etiology encompasses both genetic and environmental factors, its declining prevalence and its severity at the time of writing (2010) suggests amelioration with regard to those environmental factors.20 Most cases of PDB occur in communities of Northwestern European descent, particularly from the United Kingdom.20 Although it has been reported to affect 3% of Britons and White Americans over 50 years of age,22 it is less frequent in Africans and East Asians23,24
The presentation of PDB in East Asians appears to be accompanied with symptoms, whereas that in patients of European origin is largely symptom-free.23,24
Although the radiological presentation is broadly similar to fibrous dysplasia, PDB is generally bilateral and first presents over the age of 40 years. It classically presents with a “cotton wool” expansion of the outer table of the skull and a wholly radiopaque vertebral body. When the jaws are affected the lumen of the maxillary antrum is frequently spared.
The radiological presentation of lesions in the alveolus is similar to florid osseous dysplasia, but extends into basal bone. Note that, although classically polyostotic, at least 7 cases of monostotic Paget’s affecting the mandible alone have been reported in the literature.25 Although bone scintigraphy and serum alkaline phosphatase are sensitive screening modalities for PDB, the latter, which is raised in 86% of cases, may not be raised during the more inactive periods of the disease. It is also higher for polyostotic disease than for monostotic disease.26 Takata et al. have set out an algorithm for the diagnosis of PDB.26 Bisphosphonates are used to treat PDB.26
Although the list of complications is long and covers musculoskeletal, neurological, cardiovascular, metabolic, and neoplastic complications, the complication that may concern the oral and maxillofacial clinician most is sarcomatous change of PDB affecting the jaws.26 Cheng et al. in their synthesis of the English-language literature reported significantly more cases of osteosarcoma secondary to PDB of the jaws in contrast to osteosarcoma arising from PDB elsewhere in the skeleton and osteosarcoma arising within the jaws.27 Osteosarcoma arising from PDB of the jaws has a predilection for females and those of sub-Saharan African origin. Although the 5-year survival is poor (21%) it is better than that for osteosarcoma arising from PDB elsewhere in the skeleton, which is 5%. The survival of the former is lower than that for primary osteosarcoma arising within the jaws.27
GARDNER’S SYNDROME
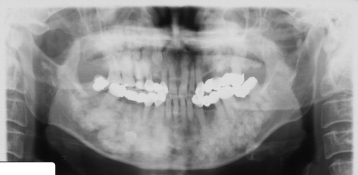
The main clinical feature of Gardner’s syndrome (GS), an autosomal disease, is familial adenomatous polyposis (FAP). GS affects only 10% of FAP.28 Any one or more of these polyps at any time can undergo malignant change. Polyps arise after 20 years of life.29 Therefore, it is important to diagnose the syndrome as early as possible. Although the taking of a good family history may assist in identification of polyps, the vigilance of the oral and maxillofacial practitioner is crucial because s/he is more likely to observe the hard tissue lesions earlier than the onset of symptoms and signs of FAP. Those hard tissue lesions affecting the jaws are osteomas (Figure 10.11), odontomas, and supernumerary and impacted teeth.30 They will be most likely to be observed as an incidental finding on a panoramic radiograph. Takeuchi et al. reported the largest series of GS cases.31 They found 23 cases of GS out of 48 cases of FAP. The average age when diagnosed was 26 years of age. They followed them for an average of 7 years and noted that in one-half of the cases the number and size of the osteomas continued to increase. Although one-third developed colonic cancer, there was no significance between malignant transformation and the extent of the jaw lesions; indeed, the 4 cases displaying widespread lesions were not associated with malignant change.
Figure 10.11. Panoramic radiograph displaying multiple osteomas in both the alveolar and basal processes of both jaws of a patient with Gardner’s syndrome (polyposis coli). The osteomas have almost completely obliterated the maxillary antrum. See Figure 17.21 for other views of this case.
Reprinted with permission from Lee BD, Lee W, Oh SH, Min SK, Kim EC. A case report of Gardner syndrome with hereditary widespread osteomatous jaw lesions. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2009;107: e68–72.
The multiple osteomas affecting the mandible of a middle-aged East Asian woman in Lee et al.’s case have a radiological presentation similar to familial gigantiform cementoma (Figure 10.11).32 The HCT of this case additionally revealed an osteoma expanding into the orbit displacing the optic nerve and the globe (eyeball) (see Figure 17.21). In addition to similar familial gigantiform cementoma-like lesions in two of her adult children there was a definite family history of abdominal tumors. On endoscopy the patient was found to have multiple intestinal polyps. The sole dental anomaly was an impacted premolar.
Fonseca et al.33, Madani et al.34, and Ramaglia et al.28 reported peripheral (periosteal) osteomas; such osteomas are rarely seen in nonsyndromic cases.
Poorly Defined Radiopacities
Poorly defined lesions are generally suggestive of aggressive disease such as malignancies or infections. This criterion by itself is not entirely decisive with regard to those jaw lesions, which frequently present as radiopacities. Fibrous dysplasia, a fibro-osseous lesion affecting the jaws, also presents with a poorly defined margin, which is central in differentiating it from another fibro-osseous lesion. Nevertheless, there is one other criterion that can assist in the identification of these aggressive lesions, the periosteal reaction. The periosteal reaction is a prominent feature of general radiology but other an expansion of the cortices it is infrequently observed in the jaws, except in regard to chronic infection and some malignant neoplasms, such as the osteosarcoma.35
OSTEOSARCOMA
Osteosarcoma is the most common of the sarcomas affecting the jaws; in an American National Cancer Database Report osteosarcoma accounted for 78% of sarcomas affecting the mandible in contrast to chondrosarcoma’s 14% and Ewing’s sarcoma’s 8%.36 Overall chondrosarcoma has a 75% 5-year survival rate, whereas both osteosarcoma and Ewing’s sarcoma is 50%. The 5-year survival rate of osteosarcoma secondary to Paget’s disease was very low, about 21%.27 Guo et al.,37 comparing Chinese, Japanese, and American databases, observed that the relative frequencies of osteosarcoma were higher in China and Japan in contrast to the United States. There were far fewer cases in people over 50 years of age in the two East Asian countries, which could be ascribed to their lower incidence of Paget’s disease. Although both chondrosarcoma and Ewing’s sarcoma were higher in the United States, chondrosarcoma first presented younger in the Chinese.37 Van Es et al. reported that the 10-year survival of osteosarcoma in their Dutch report was 59%.38
Unlike its manifestation in the extragnathic skeleton, which mainly occurs largely in the adolescent, osteosarcoma affecting the jaws occurs later in life, usually during the fourth38–40 and fifth41–43 decades. In a Nigerian report the mean age was 27 years, 31 and 23 years for the maxilla and mandible, respectively.44
Most presented as swellings. Mardinger et al. reported that the maxillary osteosarcoma was larger (13 cm2) than that affecting the mandible (8 cm2).39 Mental paresthesia was observed between 7%42 and 21%40 to 36%.39
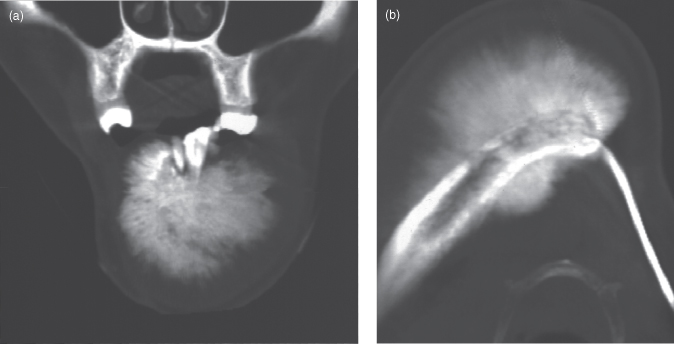
The variety in radiological presentation may merely reflect the ethnic origin of the community reported. Ogunslewe et al. reported that most cases showed a nonspecific radiolucent lesion.44 Givol et al. reported that overall 78% had poorly defined margins and 29% displayed a locular pattern.40 Forty-one percent were “mixed,” 29% were radiolucent, and 29% were radiopaque.40 Fernandes et al. reported a “sunray appearance” (Figure 10.12) in 54%42 and Givol et al. reported 48% of the cases in their synthesis with a “periosteal reaction.”40 In almost every such case, this was observed on an occlusal projection. Almost all the lesions in Givol et al.’s own case series, displaying a “periosteal reaction,” affected the posterior mandible.40
Figure 10.12. Computed tomography (CT) of a case of recurring osteosarcoma. (a) Coronal CT (bone window) exhibiting the sunburst pattern. (b) Axial CT displaying the above affecting almost the whole of the remaining body of the mandible.
Givol et al. stated that soft-tissue involvement was reported in 33% and optimally displayed on HCT.40
OSTEOMYELITIS
Osteomyelitis of the jaws frequently arises from a dental infection. Kahn et al.’s Figure 3 appears, due to its expansion of the whole bone and diffuse bone pattern (Figure 10.13), similar to a case of fibrous dysplasia.45 Petrikowski et al. reviewed 10 cases each of osteomyelitis, fibrous dysplasia, and osteosarcoma—three lesions with radiological similarities.46 They reported that the only two features that most usefully distinguish osteomyelitis are “sequestra and laminations of periosteal new bone” (Figure 10.14).46 This new bone is called an involucrum.
Figure 10.13. Panoramic radiograph displaying a diffuse sclerosing osteomyelitis arising from a carious first molar. This osteomyelitis has affected both the alveolar and basal processes, resulting in the accentuation of the mandibular canal (compare with the normal contralateral side).
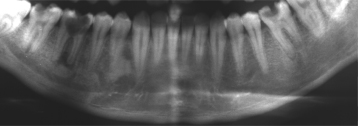
Figure 10.14. Panoramic radiograph a periosteal reaction at the lower border of the mandible apical to the root-filled first molar resulting in an involucrum. This is represented by a suggestion of laminations running approximately parallel to the lower border of the mandible giving rise to an onion-skin appearance. A wide draining tract is obvious running, through this onion-skin structure, from the periapical radiolucency to the most dependant part of the periosteal reaction. Sclerosing osteomyelitis is observed in the bone adjacent to this tooth, particularly inferiorly and distally.
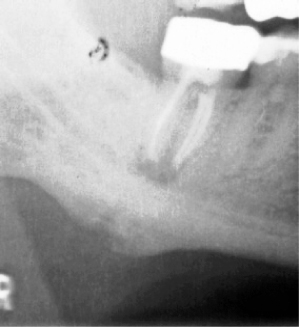
Figure 10.15 displays a case of osteomyelitis, which began in the anterior mandible, that over the years gradually involved almost the entire mandible.
Figure 10.15. A consecutive series of panoramic radiographs displaying the progression of osteomyelitis from the midline (a) to affect the entire mandible except for the condyles (c).
In a small number of cases, particularly the diffuse osteomyelitis47 may be a manifestation of SAPHO syndrome.45 This is a localized rheumatic disease with an idiopathic etiology. It presents with synovitis, acne, pustulosis, hypertelorism, and osteitis—hence its acronym, SAPHO. This syndrome is linked with spondyloarthopathies. Diagnosis requires a more general review of the patient for skin lesions and scintography to detect other skeletal lesions. If bone resorption is present it may be treated by bisphosphonates.45
BISPHOSPHONATE-ASSOCIATED OSTEONECROSIS
In addition to treating osteosarcoma, Paget’s disease of bone, and SAPHO syndrome, bisphosphonates are also central in the treatment of osteoporosis, multiple myeloma, and metastatic disease.
Although osteonecrosis of the jaw has been a long-recognized clinical phenomenon, it briefly peaked in incidence as radio-osteonecrosis until bone-saving radiotherapy was developed. Recently, it has become increasingly observed as bisphosphonate-associated osteonecrosis (BON). It is now a recognized risk of bisphosphonate therapy, particularly if intravenous and/or of long duration (over 3 years).48
It presents clinically as poor wound healing, spontaneous or postsurgical breakdown of soft tissue to expose the bone to the oral environment, and osteomyelitis. This may or may not be accompanied by pain.48
Panoramic radiography is of limited value for the assessment of BON. It displayed only a nonspecific osteolysis in all patients.49 It identified a sequestrum (Figures 10.16, 10.17) in only two-thirds of the cases identified by HCT.50 A periosteal reaction was frequently found; this was also confirmed by Bedogni et al.51 Chiandussi et al. found that the bone scan was most sensitive for identifying early-stage osteonecrosis.52 Furthermore, in such cases single photon emission computed tomography fused with computed tomography (SPECT/CT) may enhance the bone scan by distinguishing the osteonecrotic nidus from the adjacent hyperactive viable bone. The reader is reminded that bisphosphonates are part of the treatment for multiple myeloma and metastatic breast and prostrate cancers.48 Therefore, areas of hyperactivity may represent metastasis.
Figure 10.16. Panoramic radiograph and computed tomograph (CT) of a case of bisphosphonate osteonecrosis. (a) Panoramic radiograph displays affected edentulous site. The affected bone is delimited by a broad radiolucent band running parallel and above the mandibular canal. The bone above it is being sequestrated (b). The coronal CT exhibits the sequestrum.
Figures courtesy of Dr. Michele Williams, British Columbia Cancer Agency.
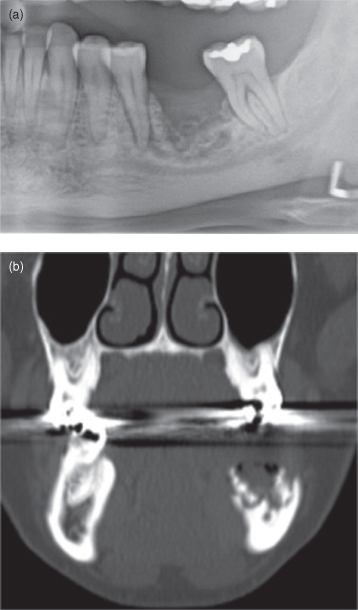
Figure 10.17. Panoramic radiograph displaying bisphosphonate osteonecrosis affecting the entire posterior alveolus of the right mandible. Posteriorly this reaches down to the mandibular canal and reaches the lower border of the mandible anteriorly.
Figure courtesy of Dr. Michele Williams, British Columbia Cancer Agency.
Both HCT’s and MRI’s definition of the extent of the osteonecrosis was invaluable for distinguishing between the osteonecrotic and osteomyelitic patterns of BON representing exposed and unexposed bone, respectively.51 The osteonecrotic pattern gave a low hypointense signal on T1-weighted and T2-weighted and inversion recovery (IR) images, suggesting a low water content, which is consistent with the paucity of cells and blood vessels. The osteomyelitic pattern was characterized by a hypointense T1-weighted, a hyperintense T2-weighted and IR images. These suggest an abundant cellular and vascular tissue with osteogenesis.51
FIBRO-OSSEOUS LESIONS
My review of the differential diagnoses of those lesions, presenting in the Hong Kong Chinese, which frequently present as radiopacities, indicated that fibro-osseous lesions (FOLs) appeared frequently. These FOLs are fibrous dysplasia, ossifying fibroma, and osseous dysplasia. As observed in Figures 10.1 and 10.2, FOLs are central in the differential diagnosis of a radiopacity affecting the jaws. Although they display a similar histopathology, a spectrum between cementoid and osteoid, their clinical and radiological presentations and treatment outcomes differ. Figure 10.18 displays the development of the nomenclature and classification of FOLs. Although it includes only those terms that appear to be currently in use, this simplified figure is still able to display the “lumping” and “splitting,” which appear to attend frequently the development of most classifications and systems of nomenclature.
Figure 10.18. Development of the nomenclature and classification of fibro-osseous lesions of the jaws.
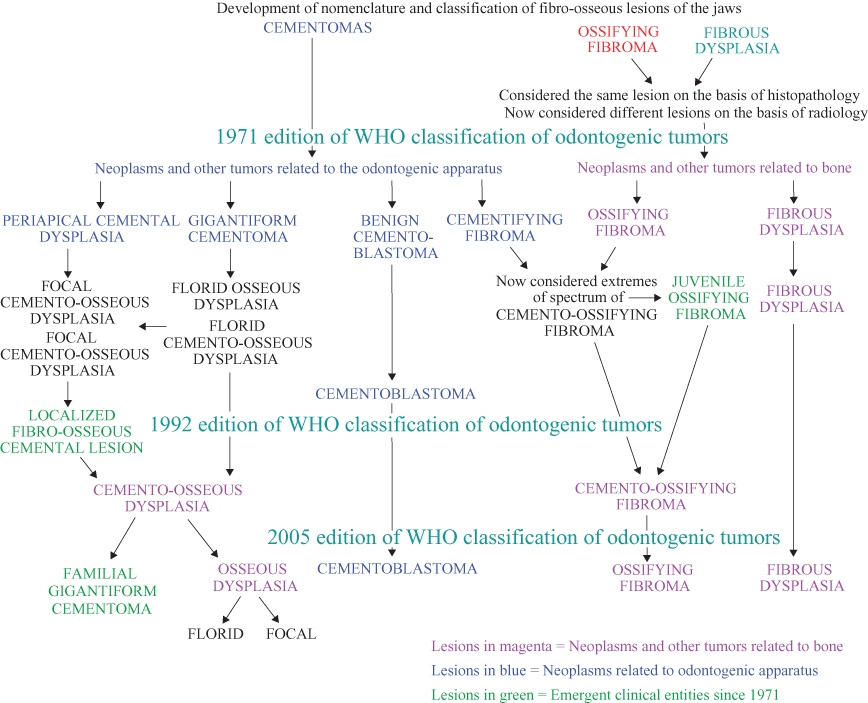
The late Charles Waldron wrote “In absence of good clinical and radiologic information a pathologist can only state that a given biopsy is consistent with a FOL. With adequate clinical and radiologic information most lesions can be assigned with reasonable certainty into one of several categories.”53 Conversely in the absence of such information Eisenberg and Eisenbud stated “pathologists today will often rightly decline to render a definitive diagnosis. … Instead, the pathologist will resort to the noncommittal designation of benign fibro-osseous lesions [their italics]. This is the only acceptable approach considering the potential for inappropriate treatment otherwise.”54 Therefore the identification or clarification of the majority of histopathologically proven FOLs affecting the jaws is made upon clinical and radiological features.
FIBROUS DYSPLASIA
Jundt55 defined fibrous dysplasia (FD) as “a genetically based sporadic disease of bone that may affect single or multiple bones … FD occurring in multiple adjacent craniofacial bones is regarded as monostotic (craniofacial FD). FD may be part of the McCune-Albright syndrome.”
FD is an important lesion affecting the maxillofacial region because it can cause severe deformity and asymmetry, and, most devastating of all, blindness.
Jundt’s basis for referring to FD as a genetically based sporadic disease of the bone is that “Mutations in the gene (GNAS I) encoding for the α-subunit of a signal transducing G-protein (Gs-α) lead to increased c-AMP production affecting proliferation and differentiation of preosteoblasts.”55
FD can present either as a local lesion (the monostotic form) or as a systemic lesion (the polyostotic form). When this last form is combined with hormonal changes, it is now the McCune-Albright syndrome (MAS), of which precocious puberty is perhaps the most striking feature.
The global distribution of reports included in the systematic review56 upon which much of the following is derived is set out in Figure 1.44 and their details in Table 10.2.
Table 10.2. Fibrous dysplasia: systematic review
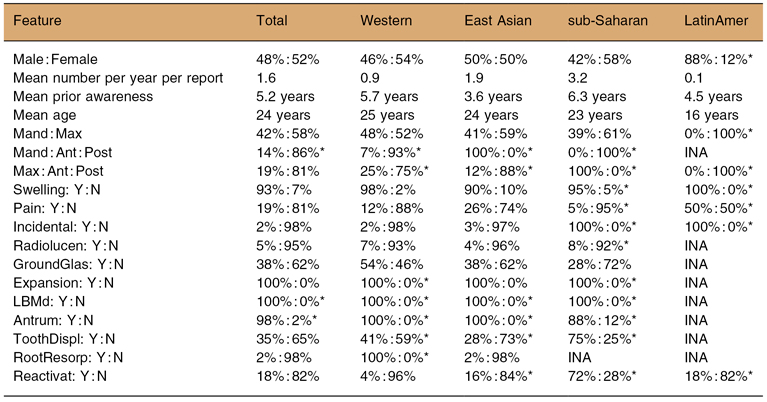
*Advises that the percentages were derived from either one report or from a synthesis of no more that 50 cases. Ant : Post, Anterior : Posterior; GroundGlas, Ground Glass; INA, Information not available; LatinAmer, Latin American; LBMd, downward expansion of the lower border of the mandible; Mand : Max, Mandible : Maxilla; Reactivat, Reactivation; subSaharan, sub-Saharan African; ToothDispl, Tooth displacement; ToothResorp, Tooth resorption; Western, predominantly Caucasian; Y : N, Yes : No.
The mean number of cases of FD per year globally was 1.6. Although this was highest for the sub-Saharan African global group and least for the Western, the difference was not significant.56
Although it is generally accepted that FD is a disease affecting children and adolescents and burns out at the end of puberty in most cases. my recent systematic review refutes this contention. Indeed, the majority first present over 20 years old (some even as late as the 8th decade) with an overall mean of 24 years (Table 10.2). In addition, the period of the patient’s prior awareness before first presentation is 5.2 years.56 Taken together, the average patient becomes first aware of his/her disease when 19 years old, precisely when it is traditionally expected to “burn out.”56 Nevertheless, almost all polyostotic cases first present early in infancy.
Therefore, if it is supposed that all FDs arise during childhood or puberty, most remain undetected only to become active or be reactivated later and thus be detected for the first time later in life. This conclusion is feasible if the classical division of FD into monostotic, polyostotic, and MAS forms is considered to reflect the timing of the mutation and, thereby, the initial size of the mass of FD precursor cells.57,58 The polyostotic form may arise in fetal life, whereas the monostotic form may arise postnatally.58 In addition to discussing how the different forms of FD arose, the two cited authorities together comprehensively discuss the genetic basis of this lesion. Some of the discussion on both topics was detailed in their earlier publications, which they cite clearly.
The systematic review demonstrated that the distribution between the sexes is almost equal, with females prevailing slightly. The mean overall age at first presentation is 24 years, ranging between 5 to 79. The second decade attracted most cases upon first presentation, 36% percent of all cases of which 67% are males. Males slightly predominate in the third decade but are in the minority in all other decades.56
The polyostotic form (with/without endocrinopathies) is easy to diagnosis because many bones are affected and it occurs in childhood, compelling the patient’s parents to seek treatment. The McCune-Albright Syndrome (MAS) is just one of a series of lesions associated with precocious puberty, which has been extensively discussed by Fahmy et al.59 They also provide an algorithm. A diagnosis of precocious puberty requires radiography of the bones of the wrist to determine the developmental age. Café-au-lait spots lead towards a diagnosis of MAS, which should then indicate a bone scan or other radiology to determine the presence of polyostotic FD.59
The monostotic form may not be so easy to diagnose. The monostotic form accounts for up to 85% of cases. Monostotic means “one bone,” which is the correct term when applied to FD affecting the mandible but is not strictly true when applied to FD affecting the maxilla. FD affecting the maxilla may involve one or more contiguous bones, such as the zygomatic (malar) and palatine bones. Therefore, “craniofacial fibrous dysplasia” has been coined for such cases and used by Jundt in the WHO’s 2005 edition.55 The maxillofacial subset of the craniofacial FD accounted for 13% in a recent systematic review.56
Although bone biopsy of FD is generally avoided in medicine, particularly in those cases where pathological fracture may be high, this appears not to be the case of FD affecting the jaws. Only one case of apparent pathological fracture and nonunion has been reported for FD affecting the mandible.60 Therefore, a biopsy, required to confirm FD histopathologically, should not be contraindicated solely for this reason. But it is a generally accepted principle that the biopsy should be deferred until all necessary imaging has been completed; otherwise, the radiological presentation may be compromised (see Figure 1.2). Biopsies are still necessary for the histopathological diagnosis of FOL, which in conjunction with a poorly defined margin of a radiopaque lesion on conventional radiography is necessary for the diagnosis of FD. Biopsy is also required to refine our understanding of the genetics of FD. It is also required for the identification of markers for future adverse conduct such as reactivation.
In spite of the fact that, at first presentation, the FD lesions in one recent report were so large that many affected all or most of the hemimandible or hemimaxilla, very few were discovered as incidental findings. Furthermore, the clinicians offered solely “fibrous dysplasia” as their provisional diagnosis only on the basis of their clinical and radiological findings.60
Only 2% are discovered as incidental findings in the systematic review; the rest presented with symptoms.56 Ninety-three percent of cases first present with swelling. Swelling was significantly more frequent in the Western global group than in the East Asian global group. Overall, 19% presented with pain.56 A higher proportion of East Asian cases present with pain in comparison to other global communities. In the jaws the maxilla is affected in 58% of cases; the Western global group approached equal distribution between the jaws. FD displays an overwhelming predilection for the posterior sextants of both jaws.56
FD affecting the face and jaws differs radiologically and histologically from that of the rest of the skeleton. FD affecting the jaws is poorly defined, according to Slootweg and Müller’s 1 mm criterion (see Chapter 1),61 whereas that of the extragnathic FD is generally well defined.62 A possible reason for this difference is that the jaws are derived from membrane and long bones are from cartilage.63 Support for this content/>
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


