Chapter 10
Liver Disease
Liver dysfunction may be attributed to a number of causes, including acquired infections and other pathologic conditions, as well as drug use. The patient with liver disease presents a significant management challenge for the dentist because the liver plays a vital role in metabolic functions, including the secretion of bile needed for fat absorption, conversion of sugar to glycogen, and excretion of bilirubin, a waste product of hemoglobin metabolism. Impairment of liver function can lead to abnormalities in the metabolism of amino acids, ammonia, protein, carbohydrates, and lipids (triglycerides and cholesterol). Many biochemical functions performed by the liver, such as synthesis of coagulation factors and drug metabolism, may be adversely affected in the dental patient with acute or chronic liver disease. Along with impaired drug metabolism, therefore, significant bleeding may be a problem to be addressed in the dental treatment plan.< ?xml:namespace prefix = "mbp" />
Cirrhosis is the consequence of long-term damage to the liver tissues. This condition is irreversible and leads to fibrosis, resulting in jaundice, ascites, and portal hypertension, as well as significant liver dysfunction. The potential causes of viral hepatitis related cirrhosis are listed in
Hepatitis
Definition
Hepatitis is inflammation of the liver that may result from infectious or other causes. Examples of hepatitis with infectious causes are viral hepatitis and that associated with infectious mononucleosis, secondary syphilis, and tuberculosis. Approximately 15,000 people in the United States die each year of cirrhosis caused by viral hepatitis.
Since the several types of hepatitis have various degrees of impact on dental treatment, each is discussed separately in subsequent sections.
Viral hepatitis is a collective term describing liver inflammation or hepatitis caused by a group of several different viruses. Three viruses, hepatitis A virus (HAV), hepatitis B virus (HBV), and hepatitis C virus (HCV), cause most cases of viral hepatitis in the United States. Because hepatitis A is transmitted primarily in unsanitary conditions, the number of annual cases has declined significantly in the United States in recent years as a result of vaccination programs and food safety efforts.
Unlike HAV hepatitis, infections by HBV and HCV are bloodborne and/or often persist for years, resulting in ongoing (chronic) but usually asymptomatic liver inflammation and, in some cases, scarring (cirrhosis) that leads to liver failure and/or liver cancer. Chronic hepatitis is a major cause of liver cancer and chronic liver disease globally and in the United States.
Epidemiology
Acute viral hepatitis is a common disease that affects 0.5% to 1% of persons in the United States each year. The annual incidence of acute hepatitis has been decreasing steadily since 1990, largely because of the use of hepatitis A and B vaccines and decrease in high-risk behaviors. In recent population-based surveys, viral causes of acute hepatitis were HAV in 37%, HBV in 45%, and HCV in 18% of cases. Worldwide, 480 million to 540 million persons are living with chronic viral hepatitis, with 350 million to 370 million infected with HBV and 130 million to 170 million infected with HCV.
Chronic hepatitis causes considerable morbidity. Globally, an estimated 78% of primary liver cancer and 57% of liver cirrhosis are caused by chronic viral hepatitis, and about 1 million deaths from viral hepatitis occur each year.
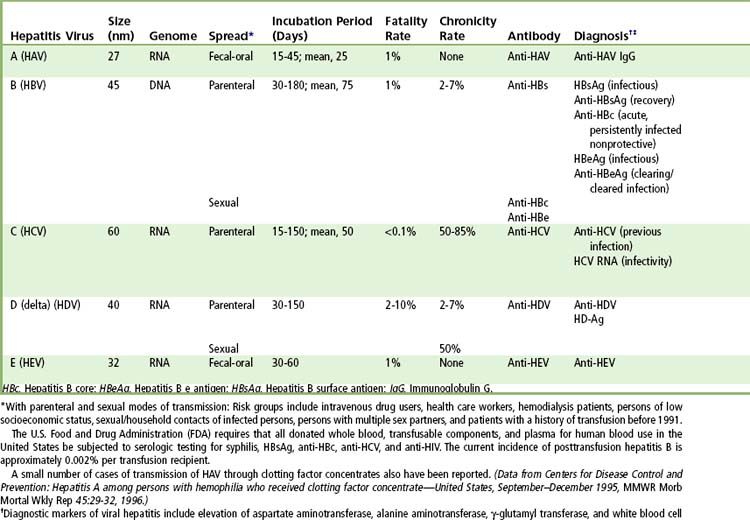
The clinical manifestations of the five forms of viral hepatitis are quite similar, and the diseases can be distinguished from each other only by serologic assays.
Pathophysiology
The pathogenesis of the liver injury in viral hepatitis is not well understood. None of the five primary agents seems to be directly cytopathic, at least at levels of replication found during typical acute and chronic hepatitis. The timing and histologic appearance of hepatocyte injury in viral hepatitis suggest that immune responses, particularly cytotoxic T cell responses to viral antigens expressed on hepatocyte cell membranes, may be the major effectors of injury. Other proinflammatory cytokines, natural killer cell activity, and antibody-dependent cellular cytotoxicity also may play modulating roles in cell injury and inflammation during acute hepatitis virus infection. Recovery from hepatitis virus infection usually is accompanied by the appearance of rising titers of antibody against viral envelope antigens, such as anti-HAV, anti-HBs, anti-HCV-E1 and anti-HCV-E2, and anti-HEV; these antibodies may provide at least partial immunity to reinfection.
Clinical Manifestations
The course of acute hepatitis is highly variable and ranges in severity from a transient, asymptomatic infection to severe or fulminant disease. The disease may be self-limited with complete resolution, run a relapsing course, or lead to chronic infection. In a typical, clinically apparent course of acute resolving viral hepatitis, the incubation period ranges from 2 to 20 weeks, as determined largely on the basis of the viral etiologic agent and exposure dose. During this phase, virus becomes detectable in blood, but serum aminotransferase and bilirubin levels are normal, and antibody is not detected.
The preicteric phase of illness is marked by the onset of nonspecific symptoms such as fatigue, nausea, poor appetite, and vague right upper quadrant pain. Virus-specific antibody first appears during this phase. The preicteric phase typically lasts 3 to 10 days but may be of longer duration and even constitute the entire course of illness in patients with subclinical or anicteric forms of acute hepatitis. Viral titers generally are highest at this point, and serum aminotransferase levels start to increase.
The onset of production of dark urine marks the icteric phase of illness, during which jaundice appears and symptoms of fatigue and nausea worsen. Typically, acute viral hepatitis rarely is diagnosed correctly before the onset of jaundice. If jaundice is severe, stool color lightens, often in association with pruritus. Other manifestations may include anorexia, dysgeusia, and weight loss. Physical examination usually shows jaundice and hepatic tenderness. In more severe cases, hepatomegaly and splenomegaly may be present. Serum bilirubin levels (total and direct) rise, and aminotransferase levels generally are higher than 10 times the upper limit of normal, at least at the onset. During the icteric, symptomatic phase, levels of hepatitis virus begin to decrease in serum and liver.
The duration of clinical illness is variable; it typically lasts 1 to 3 weeks. Recovery is first manifested by return of appetite and is accompanied by resolution of the serum bilirubin and aminotransferase elevations and clearance of virus. Convalescence can be prolonged, however, before full energy and stamina return. Neutralizing antibodies usually appear during the icteric phase and rise to high levels during convalescence.
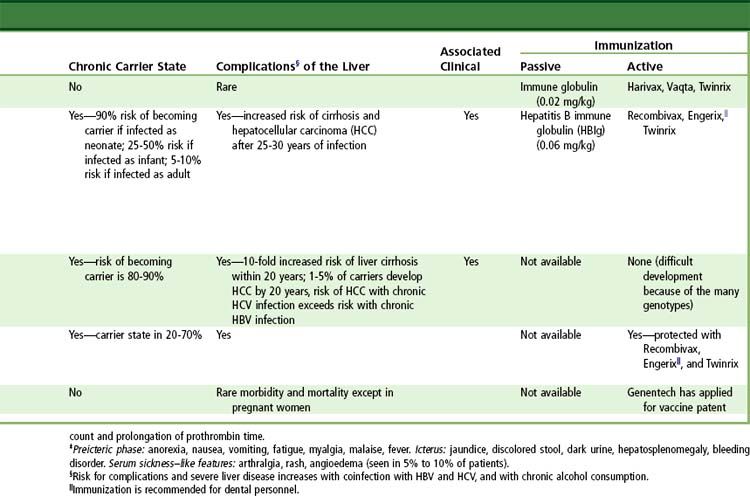
Complications of acute viral hepatitis include chronic infection, fulminant hepatic failure, relapsing or cholestatic hepatitis, and extrahepatic syndromes. Chronic hepatitis, generally defined as illness of at least 6 months’ duration, develops in approximately 2% to 7% of adults with hepatitis B and in 50% to 85% of adults with hepatitis C. Hepatitis B, C, and D infections are said to be chronic if viremia persists for more than 6 months, but chronicity can be suspected if viremia persists for 3 months after the onset of symptoms.
Acute liver failure or fulminant hepatitis occurs in 1% to 2% of patients with symptomatic acute hepatitis, perhaps most commonly with hepatitis B and hepatitis D and least commonly with hepatitis C. The disease is called fulminant if evidence of hepatic encephalopathy appears; however, the initial symptoms (changes in personality, aggressive behavior, or abnormal sleep patterns) may be subtle or misunderstood. The most reliable prognostic factor in acute hepatic failure is the degree of prolongation of the prothrombin time; other signs of poor prognosis are persistently worsening jaundice, ascites, and decreases in liver size. Serum aminotransferase levels and viral titers have little prognostic value and often decrease with worsening hepatic failure.
In 10% to 20% of patients with acute hepatitis, a serum sickness–like syndrome marked by variable combinations of rash, hives, arthralgias, and fever develops during the preicteric phase. This immune complex–like syndrome often is mistakenly attributed to other illnesses until the onset of jaundice, at which time the fever, hives, and arthralgias quickly resolve. Other extrahepatic manifestations of acute hepatitis are uncommon but may include severe headaches, encephalitis, aseptic meningitis, seizures, acute ascending flaccid paralysis, nephrotic syndrome, and seronegative arthritis.
Diagnosis
Serologic tests are adequate for the diagnosis of acute viral hepatitis (
TABLE 10-2 Serologic Diagnosis of Acute Hepatitis
| Diagnosis | Screening Assays | Supplemental Assays |
|---|---|---|
| Hepatitis A | IgM anti-HAV | None needed |
| Hepatitis B | HBsAg, IgM anti-HBc | HBeAg, anti-HBe, HBV DNA |
| Hepatitis C | Anti-HCV by EIA | HCV RNA by PCR assay; anti-HCV by immunoblot analysis |
| Hepatitis D | HBsAg | Anti-HDV |
| Hepatitis E | History | Anti-HEV |
| Mononucleosis | History, white blood cell differential counts | Heterophile antibody |
| Drug-induced hepatitis | History |
EIA, Enzyme immunoassay; HAV, Hepatitis A; HBc, Hepatitis B core; HBeAg, Hepatitis B e antigen; HBsAg, Hepatitis B surface antigen; HBV, Hepatitis B; HCV, Hepatitis C; HDV, Hepatitis D; HEV, Hepatitis E; IgM, Immunoglobulin M; PCR, Polymerase chain reaction.
Treatment
Although antiviral therapies have not been proved to be effective in prospective controlled trials, recent uncontrolled studies suggest that such therapies may be effective in acute hepatitis B and hepatitis C (see further on). However, several recommendations are applicable to the management of all patients with acute hepatitis. Bedrest and sensible nutrition are appropriate for patients who are symptomatic and jaundiced. Alcohol should be avoided until after convalescence. Sexual contacts should be limited until partners receive prophylaxis. In hepatitis A, all household contacts should be given immune globulin, and initiation of HAV vaccination is appropriate. In hepatitis B, family members should be vaccinated, and hepatitis B immune globulin (HBIG) also should be given to recent sexual contacts. Patients in whom any signs of fulminant hepatic failure develop (prolongation of the prothrombin time, personality changes, confusion) should be considered for antiviral therapy and be evaluated quickly for possible liver transplantation (see
Hepatitis A
Epidemiology
Hepatitis A is highly contagious and is spread largely by the fecal-oral route, especially when sanitary conditions are poor. Hepatitis A has been decreasing in frequency in the United States but is still an important cause of acute liver disease worldwide. Acute hepatitis A can occur in sporadic as well as epidemic forms. Investigation of the source of infection reveals that most cases are due to direct person-to-person exposure and, to a lesser extent, to direct fecal contamination of food or water. Consumption of shellfish from contaminated waterways is a well-known but quite uncommon means of acquiring hepatitis A. Rare instances of spread of hepatitis A by blood transfusions and administration of pooled plasma products have been described. Groups at high risk for acquiring hepatitis A include travelers to developing areas of the world, children in day care centers (and secondarily their parents), men who have sex with men, injection drug users, hemophiliacs given plasma products, and residents and staff of institutions.
Pathophysiology
HAV is a small RNA virus that belongs to the family Picornaviridae (genus Hepatovirus). The viral genome is 7.5 kilobases (kb) in length and has a single large open reading frame that encodes a polyprotein with structural and nonstructural components. The virus replicates largely in the liver and is assembled in the hepatocyte cytoplasm as a 27-nm particle with a single RNA genome and an outer capsid protein (HAVAg). The virus is secreted into bile and, to a lesser extent, serum. The highest titers of HAV are found in stool (106 to 1010 genomes per gram) during the incubation period and early symptomatic phase of illness.
Clinical Manifestations
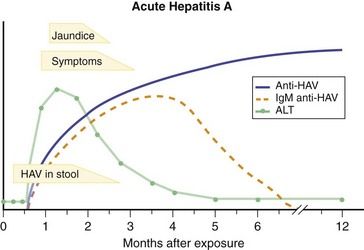
The clinical course of typical acute hepatitis A (
Diagnosis
The diagnosis of acute hepatitis A is made by detection of IgM anti-HAV in the serum of a patient with the clinical and biochemical features of acute hepatitis. Testing for total anti-HAV is not helpful in diagnosis but is used to assess immunity to hepatitis A.
Prevention
A safe and effective HAV vaccine is available and recommended for all children 1 year of age and older and for persons at increased risk for acquiring hepatitis A, including travelers to endemic areas of the world, men who have sex with men, and injection drug users. HAV vaccine also is recommended for all patients with chronic liver disease and recipients of pooled plasma products, such as hemophiliacs. Two formulations of HAV vaccine are available in the United States; both consist of inactivated hepatitis A antigen purified from cell culture. Havrix (GlaxoSmithKline, Philadelphia, Pennsylvania) is recommended to be given as two injections 6 to 12 months apart in an adult dose of 1440 enzyme-linked immunosorbent assay (ELISA) units (1.0 mL) and in a pediatric (2 to 18 years of age) dose of 720 ELISA units (0.5 mL). Vaqta (Merck, West Point, Pennsylvania) is recommended to be given as two injections 6 to 18 months apart in an adult dose of 50 U (1.0 mL) and in a pediatric dose (1 to 18 years) of 25 U (0.5 mL). A combination HAV-HBV vaccine (Twinrix) (GlaxoSmithKline) also is available; this preparation is recommended for adults who require vaccination against both forms of hepatitis and is given in a three-injection schedule at 0, 1, and 6 months after exposure. HAV vaccines have an excellent safety record, with serious complications occurring in less than 0.1% of recipients. Seroconversion rates after HAV vaccine are greater than 95% but are lower among patients with chronic liver disease, human immunodeficiency virus (HIV) infection, and other conditions of immunocompromise.
Hepatitis B
Epidemiology
Hepatitis B is spread predominantly by the parenteral route or by intimate personal contact. It is endemic in many areas of the world, such as Southeast Asia, China, Micronesia, and sub-Saharan Africa. Lesser rates occur in the Indian subcontinent and the Middle East. In the United States, hepatitis B is the most common cause of acute hepatitis, and chronic HVB infection affects approximately 0.5% of the population. Investigations of the source of infection reveal that most adult cases are due to sexual or parenteral contact. Hepatitis B is common in injection drug users, heterosexual persons with multiple sexual partners, and men who have sex with men. Blood transfusion and plasma products are now rarely infectious for hepatitis B because of the institution of routine screening of blood donations for hepatitis B surface antigen (HBsAg) and antibody to hepatitis B core antigen (HBcAg), anti-HBc (IgG antibody). Maternal-infant spread of hepatitis B is another important mode of transmission not only in endemic areas of the world but also in the United States among immigrants from these endemic areas. Routine screening of pregnant women and prophylaxis of newborns are now recommended. Intrafamilial spread of hepatitis B also can occur, although the mode of spread in this situation is not well defined. Unfortunately, lack of attention to standard (formerly universal) precautions and aseptic technique, especially the cleaning of shared medical devices, remains an important root cause of small outbreaks and sporadic cases of acute hepatitis B.
Pathophysiology
HBV is a double-shelled, enveloped DNA virus belonging to the family Hepadnaviridae (genus Orthohepadnavirus). The viral genome consists of partially double-stranded DNA, is 3.2 kb in length, and possesses four partially overlapping open reading frames that encode the genes for HBsAg (S gene), HBcAg (C gene), HBV polymerase (P gene), and a small protein, HBxAg, that seems to have transactivating functions (X gene). The virus infects only humans and higher apes and replicates predominantly in hepatocytes and perhaps to a lesser extent in stem cells in the pancreas, bone marrow, and spleen. The clinical course of hepatitis B with serologic markers is depicted in
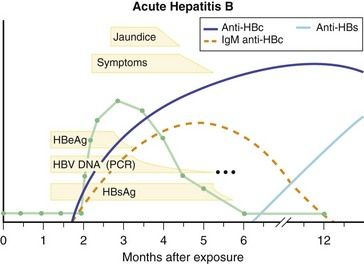
FIGURE 10-2 Serologic course of acute hepatitis B. HBc, Hepatitis B core; HBeAg, Hepatitis B e antigen; HBs, Hepatitis B surface; HBsAg, Hepatitis B surface antigen; HBV, Hepatitis B virus; PCR, Polymerase chain reaction.
(From Goldman L, Ausiello D, editors: Cecil textbook of medicine, ed 23, Philadelphia, 2008, Saunders.)
Clinical Manifestations
The typical course of acute, self-limited hepatitis B begins with an incubation period of 30 to 150 days (mean, 75 days). During the incubation period, HBsAg, HBeAg, and HBV DNA (see
Diagnosis
The diagnosis of acute hepatitis B can be made on the basis of finding HBsAg in the serum of a patient with the clinical and biochemical features of acute hepatitis. HBsAg also may be present as a result of chronic hepatitis B or the carrier state. Also, a patient with acute hepatitis and HBsAg in serum may have chronic hepatitis and a superimposed form of acute injury, such as acute hepatitis A or D or drug-induced liver disease. Testing for IgM anti-HBc (IgG antibody) is therefore helpful, because this antibody arises early and is lost within 6 to 12 months of the onset of illness. Testing for HBeAg, anti-HBe, HBV DNA, and anti-HBs generally is not helpful in the diagnosis of hepatitis B but may be valuable in assessing prognosis. Persons who remain HBV DNA– or HBeAg-positive (or both) at 6 weeks after the onset of symptoms are likely to be developing chronic hepatitis B. Loss of HBeAg or HBV DNA is a favorable serologic finding. Similarly, loss of HBsAg plus the development of anti-HBs denotes recovery.
Hepatitis B also is an important cause of fulminant hepatitis. Factors associated with severe outcomes of acute hepatitis B include advanced age, female sex, and perhaps certain strains of virus. Some variants of HBV lack the ability to produce HBeAg because of a mutation in the precore region of the viral genome. These precore or HBeAg-negative mutants are associated with atypical forms of acute and chronic hepatitis B. Several clusters of severe or fulminant hepatitis B have been associated with infection with the HBeAg-negative forms of virus.
Prevention
Vaccination against HBV is recommended for all newborns, children, and adolescents, as well as adults at risk for acquiring HBV, including health care and public safety workers with exposure to blood, injection drug users, men who have sex with men, persons at risk for sexually transmitted infections, people traveling internationally to endemic regions, and persons with close contact with patients who have chronic hepatitis B. Two formulations of HBV vaccine are available in the United States; both are made by recombinant techniques using cloned HBV S gene expressed in Saccharomyces cerevisiae. For adults, the recommended regimen is three injections of 1.0 mL (20 µg of Energix-B [GlaxoSmithKline]
Postexposure prophylaxis with HBIG is recommended at birth for infants born to infected mothers and for persons with percutaneous exposure to a patient with hepatitis B. A single dose of HBIG (0.5 mL in newborns of infected mothers and 0.06 mL/kg in other settings and in adults) should be given as soon as possible after exposure, and HBV vaccination should be started immediately. HBIG is unlikely to provide benefit if the time since exposure is longer than 14 days; vaccine alone can be used in these circumstances. For patients who have had sexual or household contact with a person who has chronic hepatitis B, vaccination alone is appropriate; HBIG is recommended in addition for sexual exposure to a person with acute hepatitis B.
Treatment
The use of antiviral therapy for acute hepatitis B is controversial. Regimens of interferon alfa and lamivudine are established therapies for chronic hepatitis B, but they have not been adequately evaluated for acute infection. In a small study, interferon alfa did not decrease the rate of chronicity or speed recovery. Uncontrolled observations with use of lamivudine in patients with fulminant and severe hepatitis B, however suggest that this therapy may ameliorate the course of infection. Because of the safety of lamivudine therapy and the unpredictable and potentially serious outcome with severe cases of acute hepatitis B, therapy with lamivudine (100 mg daily until the disease has resolved and results of HBsAg testing have become negative) is prudent for patients with signs or symptoms of fulminant liver disease (rising prothrombin time, severe jaundice), particularly if high levels of HBV DNA are present. Management of acute hepatitis B also should focus on avoidance of further hepatic injury and prophylaxis of contacts. The patient should be monitored by repeat testing for HBsAg and alanine aminotransferase levels 3 to 6 months later to determine whether chronic hepatitis B has developed.
Prognosis
Chronic hepatitis B develops in 2% to 7% of adults infected with HBV, more commonly in men and in immunosuppressed persons. The risk of chronic infection also correlates with age. It occurs in 90% of newborns infected with HBV, in approximately 30% of infants, but in less than 10% of adults. Chronic hepatitis B is still the third or fourth most common cause of cirrhosis in the United States and is an important cause of liver cancer.
Hepatitis C
Epidemiology
Hepatitis C is spread predominantly by the parenteral route. At highest risk for contracting this disease are injection drug users and persons with multiple parenteral exposures. Sexual transmission of hepatitis C is uncommon. Prospective follow-up evaluation of spouses and sexual partners of patients with chronic hepatitis C shows the risk for sexual transmission to be low (less than 1% per year of exposure). Maternal-infant spread occurs in approximately 5% of cases, usually in infants whose mothers have high levels of HCV RNA in serum and have experienced a protracted delivery or early rupture of membranes. Other potential sources of HCV are needlestick accidents and either contamination or inadequate sterilization of reusable needles and syringes. There remain, however, many persons with chronic hepatitis C who were infected with the virus by these means in the past. Current studies of acute hepatitis C indicate that more than 60% of cases are attributable to injection drug use; 15% to 20% to sexual exposure (usually involving multiple sexual partners); and only a small proportion to maternal-infant spread, needlestick accidents, and iatrogenic causes. Approximately 10% of cases are not associated with any history of potential exposure and remain unexplained.
Pathophysiology
HCV is an RNA virus that belongs to the family Flaviviridae (genus Hepacivirus). HCV originally was identified by molecular techniques, and the virus has not been well visualized. HCV probably circulates as a double-shelled enveloped virus, 50 to 60 nm in diameter. The genome is a positive-stranded RNA molecule; it is approximately 9.6 kb in length and contains a single large open reading frame encoding a large polyprotein that is posttranslationally modified into three structural and several nonstructural polypeptides. The structural proteins include two highly variable envelope antigens, E1 and E2, and the relatively conserved nucleocapsid protein C. HCV replicates largely in the liver and is detectable in serum in titers of 105 to 107 virions/mL during acute and chronic infection.
Clinical Manifestations
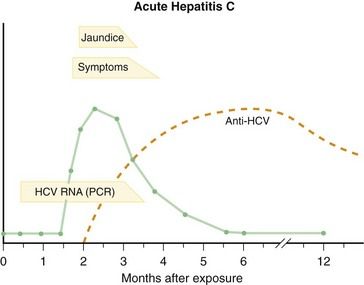
The clinical course of acute hepatitis C (
Diagnosis
Diagnosis of acute hepatitis C generally is based on detection of anti-HCV in serum in a patient with the clinical and biochemical features of acute hepatitis. The clinical course of hepatitis C with serologic markers is depicted in
Prevention
At present, there are no means of prevention of hepatitis C other than avoidance of high-risk behaviors and appropriate use of standard precautions. Injection drug use is currently the most common cause of newly acquired cases of hepatitis C. In this regard, needle exchange programs and education regarding the risks of drug use, including intranasal cocaine, and the risk of transmission from shared injection equipment are important.
Accidental needlestick exposure is perhaps the most frequent issue in prevention of transmission. At present, neither immune globulin nor preemptive therapy with antiviral agents or interferon is recommended in this situation. Monitoring by means of determination of aminotransferase levels and HCV RNA and anti-HCV testing (at baseline and again at 1 and 6 months after exposure) is appropriate. This approach allows for early intervention and treatment.
Treatment
Therapy with peginterferon alfa and ribavirin
Prognosis
The major complication of acute hepatitis C is the development of chronic hepatitis. The clinical course depicted in
Hepatitis D
Epidemiology
Hepatitis D is linked to hepatitis B, and consequently its epidemiology is similar. HDV can be spread by the parenteral route and through sexual contact. People at greatest risk are chronic carriers of hepatitis B and persons who have repeated parenteral exposures. In the United States and Western Europe, delta hepatitis is most common in injection drug users and, before routine screening of blood donations, recipients of blood products, including persons with hemophilia and thalassemia. Delta hepatitis is endemic in the Amazon basin and central Africa and is common in some European and Mediterranean countries, including southern Italy, Greece, and eastern Europe.
Pathophysiology
The hepatitis delta virus is a unique RNA virus that requires HBV for replication. The viral genome is a short, 1.7-kb circular single-stranded molecule of RNA that has a single open reading frame and a highly conserved, nontranslated region that resembles the self-replicating element of viroids. The single open reading frame encodes delta antigen, and RNA editing can vary the size of the molecule to produce either a small (195 amino acids) or large (214 amino acids) delta antigen. The small delta antigen promotes the replication of HDV RNA; the large delta antigen promotes viral assembly and secretion into serum as the mature 36-nm delta viral particle.
Clinical Manifestations
Delta hepatitis occurs in two clinical patterns termed coinfection and superinfection. Delta coinfection is the simultaneous occurrence of acute HDV and acute HBV infections. Clinically and serologically it resembles acute hepatitis B but may manifest a second elevation in aminotransferase levels associated with the period of delta virus replication. The diagnosis of acute delta coinfection can be made in a patient with clinical features of acute hepatitis who has HBsAg, anti-HDV, and IgM anti-HBc in serum. Immunoassays for anti-HDV are commercially available and reliable, although antibody may appear late during the illness. In patients suspected of having delta hepatitis, repeat testing for anti-HDV during convalescence is appropriate.
Diagnosis
Acute delta superinfection is the occurrence of acute HDV infection in a person with chronic hepatitis B or the HBsAg carrier state. The diagnosis of superinfection can be made in a patient with clinical features of acute hepatitis who has HBsAg and anti-HDV but no IgM anti-HBc in serum. Superinfection with HDV is more frequent than coinfection and is far more likely to lead to chronic delta hepatitis. Other tests that are helpful in making the diagnosis of ongoing HDV infection are determinations of serum HDV RNA (detectable by PCR assay) and HDV antigen (detectable by immunoblot analysis); both of these tests are currently research assays and not standardized. Delta antigen also can be detected readily in liver biopsy specimens11 with immunohistochemical staining.
Prevention
Delta hepatitis can be prevented by preventing hepatitis B. The severity of delta hepatitis is another compelling rationale for routine hepatitis B vaccination in areas of the world where delta hepatitis is endemic. There are no means of prevention of delta hepatitis in a person who is already an HBsAg carrier; in this situation,
Treatment
No specific therapies are available for acute delta hepatitis. Lamivudine and other anti-HBV agents are ineffective against HDV replication. Most cases of acute coinfection resolve; patients with superinfection should be treated when it is clear that chronic delta hepatitis has supervened.
Hepatitis E
Epidemiology
Hepatitis E is responsible for epidemic and endemic forms of non-A, non-B hepatitis that occur in less-developed areas of the world. Large outbreaks have been described from India, Pakistan, China, northern and central Africa, and Central America. In studies from India and Egypt, hepatitis E has accounted for a high proportion of cases of sporadic acute hepatitis. In the United States and Western Europe, hepatitis E is rare, with most cases being imported or caused by zoonotic spread from swine or rats that harbo/>
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses