Dental Implant Maintenance
At the conclusion of this chapter, the reader will be able to:
 . Describe the peri-implant attachment apparatus and compare it with the periodontium of natural teeth.
. Describe the peri-implant attachment apparatus and compare it with the periodontium of natural teeth.
 . Describe the clinical and radiographic characteristics of healthy dental implants.
. Describe the clinical and radiographic characteristics of healthy dental implants.
 . List procedures commonly performed at dental implant recall appointments.
. List procedures commonly performed at dental implant recall appointments.
 . Describe the instrumentation used for dental implant débridement.
. Describe the instrumentation used for dental implant débridement.
 . Discuss the use of adjunctive antimicrobial therapy in implant maintenance.
. Discuss the use of adjunctive antimicrobial therapy in implant maintenance.
 . List indications for surgical treatment of peri-implantitis.
. List indications for surgical treatment of peri-implantitis.
 . Determine the appropriate recall interval for dental implant patients.
. Determine the appropriate recall interval for dental implant patients.
Periodontitis Versus Peri-Implantitis
The dentogingival complex associated with natural teeth consists of the gingival sulcus, the junctional epithelium, and the connective tissue attachment. The connective tissue fibers are oriented perpendicular to the long axis of the tooth and insert into the root surface cementum.1–3 Although a sulcus and a junctional epithelium are associated with dental implants, the connective tissue fibers are oriented parallel to the long axis of the implant and the attachment is an adhesion.4,5 Whether the difference in the nature of connective tissue attachment results in greater risk of attachment loss for implants is not known. For natural teeth, animal studies have shown no difference in the risk of breakdown between connective tissue and junctional epithelial attachments.6
The composition of the associated microbial flora is similar for natural teeth and implants.7,8 Periodontal pathogens are reduced but not eliminated in completely edentulous patients, leaving these patients at some risk for colonization of implant surfaces.9,10 A major etiologic factor in periodontitis is the formation of a biofilm harboring pathogenic bacteria, and this is also true for peri-implantitis. Bacterial colonization of implant abutments is similar for zirconia and titanium abutments.11
Peri-implantitis is an inflammatory process that affects the tissues around an osseointegrated implant in function, resulting in loss of supporting bone. Peri-implant mucositis is a condition of reversible inflammatory changes of the peri-implant soft tissues in the absence of bone loss.12,13 The prevalence of peri-implantitis has been reported to be as low as approximately 10% to as high as 47%; the prevalence of peri-implant mucositis is generally greater, ranging from 32% to 80%.14–17
Periodontal and peri-implant bone turnover is a balanced dynamic process that involves resorption and formation, controlled and influenced by the local production of cytokines, with a wide range of inflammatory, hemopoietic, metabolic, and immunomodulatory properties.18,19 Peri-implant microbial contamination or infection (bacteria and viruses) elicits an immune response regulated by key cytokines (i.e., tumor necrosis factor alpha [TNF-alpha], interleukin 1 beta [IL-1 beta], transforming growth factor [TGF-beta], IL-10) that control the progression or suppression (or both) of the inflammatory response. Overproduction of proinflammatory cytokines, released by monocytes/macrophages and T cells in response to a microbial challenge, can lead to the breakdown of the periodontal or peri-implant tissues.20 Studies have shown that the subgingival microbiota around implants affected by pocketing and bone loss had high levels of periodontal pathogens, and periodontally involved teeth in partially edentulous patients may serve as microbial reservoirs.21,22 In addition, surgical trauma is partly responsible for an early hyperinflammatory response, which is characterized by the release of both TNF-alpha and IL-1 beta.23 On the other hand, ions released from dental implants can stimulate peripheral blood mononuclear cells (PBMCs) to produce IL-1 beta and TNF-alpha in vitro.24 Commercially pure titanium and titanium alloys have been associated with the production of other cytokines such as IL-6 and IL-18.25
IL-1 beta and TNF-alpha appear to play major roles in mediating the inflammatory response in the pathogenesis of many chronic inflammatory diseases, such as rheumatoid arthritis.26,27 Elevated levels of IL-1 beta are present in the gingival crevicular fluid (GCF) in the course of periodontitis and peri-implant inflammation.28,29 IL-1 beta is produced primarily by monocytes but may be produced by other nucleated cells in response to injury.24 TNF-alpha, a cytokine with some functions similar to those of IL-1 beta, has been detected in sites affected by periodontitis.30 Moreover, TNF-alpha and IL-1 beta act synergistically to initiate the cascade of inflammatory mediators.31 IL-6 has proinflammatory effects and is responsible for collagen resorption of gingival tissues,32 whereas IL-10 is an inhibitor of inflammation.33 Other cytokines, such as IL-12, appear to induce the secretion of interferon gamma [IFN-gamma] from activated T cells and natural killer (NK) cells,34 and IL-8 acts as a potent chemoattractant for neutrophils in gingival tissues.35
The continuous balance that exists between the host immune response and potential subgingival pathogens (bacteria and viruses) determines the clinical condition, not only around teeth, but also around osseointegrated dental implants. Nowzari et al.36 analyzed the production of cytokines around clinically healthy teeth and dental implants and examined their relationship to putative periodontal pathogens. Although no specific microbiologic profile was observed, teeth allowed for more colonization by Porphyromonas gingivalis, Tannerella forsythia, and Fusobacterium spp. Microscopic structural differences between dental and implant surfaces could account for this finding.
No information is available on the detection of human cytomegalovirus (HCMV) around healthy dental implants. In contrast to implants, HCMV is less often detected around periodontally healthy teeth. Nowzari et al.36 did not detect HCMV around healthy dental implants using nested polymerase chain reaction (PCR). The absence of prominent inflammation could help explain this result. Studies addressing a potential pathologic role of HCMV around implants are needed.
A tendency toward more cytokine production was observed around implants in contrast to teeth, but a specific explanation for this finding is not available.37 An implant may act as a foreign object and result in cytokine secretion. This raises the issue of an immune response against the chemical components of the implant. Perala et al.38 indicated that dental implant surfaces may lead to an activation of human peripheral blood mononuclear cells for the secretion of IL-1 beta and TNF-alpha.
Titanium particles in vitro have been shown to influence the release of IL-2, TNF-alpha, and IL-6.39 In an in vitro controlled experiment, Sedarat et al.40 exposed titanium implants to an environment similar to in vivo conditions and measured 16 (± 5) ng/cm2/day dissolution of titanium and titanium alloy over 96 days. The dissolution of titanium and titanium alloy and the ions released by the atomic process of biodegradation can explain, at least in part, the presence of cytokines where no microbial pathogens could be detected. The other contents of commercially pure titanium implants (e.g., carbon, iron, nitrogen, oxygen, and hydrogen) require further evaluations.
Patients who had positive results for at least one of the 11 microorganisms tested by culture had higher levels of IL-1 beta, TNF-alpha, IL-10, and IL-8 at teeth and implant sites. Virulence factors from periodontopathic bacteria (e.g., P. gingivalis) are potent stimulants for the secretion of proinflammatory cytokines (IL-1 beta, TNF-alpha) and the subsequent activation of matrix metalloproteinases (e.g., MMP-2) and other collagenases from gingival fibroblasts.41 Because active IL-1 beta and TNF-alpha mediate a variety of biologic functions, including osteoclast activation,42 leukocyte recruitment, and excessive production of MMPs,43 the overproduction of these cytokines at some point could lead to bone resorption and collagen degradation. In addition, the production of IL-8 in gingival tissues is an important recruitment mechanism for polymorphonuclear neutrophils (PMNs) and constitutes a first line of immune defense. PMNs produce IL-1 beta in response to bacterial challenge and act in a paracrine function, preventing apoptosis and increasing the phagocytic activity of other PMNs.44 Low PMN counts in clinically healthy gingival tissues are a common finding in histological analysis of teeth and implant sites.45 The balance between this innate response and the bacterial challenge is partly responsible for maintaining the health of gingival tissues. Nevertheless, although previous studies have reported that cytokine activity seems to be relevant for alveolar bone resorption and destruction of collagen,46,47 periodontal research to date has not yet established any particular cytokine profile that could help predict disease progression. Moreover, no known cytokine level threshold can differentiate between a stable site and the initiation of a pathologic process in periodontal and peri-implant tissues.
Characteristics of Healthy, Stable Dental Implants
Clinical findings for healthy dental implants include firm, pink peri-implant mucosa, shallow probing depths (3 mm or less); absence of bleeding on gentle probing, absence of purulence or suppuration, and lack of response to percussion.48 Implant-supported restorations should provide comfortable function and appropriate esthetics. Radiographic bone levels are generally located at the first thread of the implant.49 However, the practitioner must keep in mind that standard dental radiographs are two dimensional and do not generally provide information about buccal, lingual, or palatal bone levels. Buccal, lingual, and palatal attachment levels are assessed by gentle probing (Figures 10-1 to 10-5).
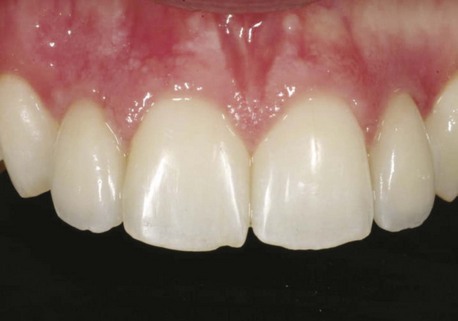
Figure 10-1 Healthy implants #7 and #10 after 6 years.
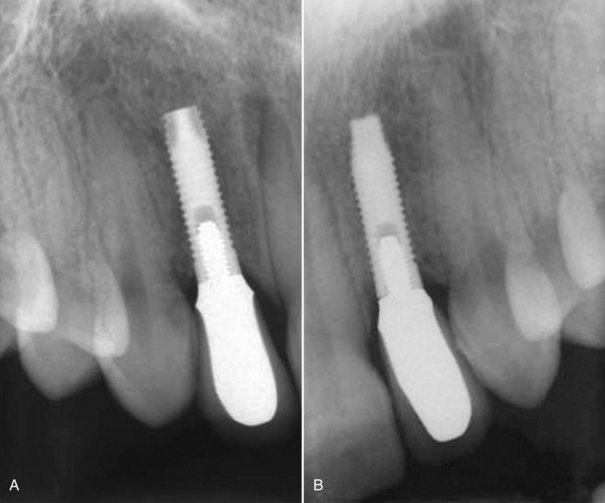
Figure 10-2 Healthy implants #7 and #10 after 6 years.
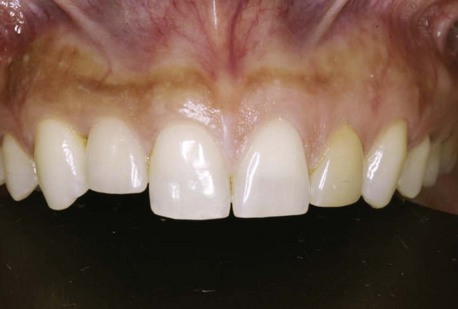
Figure 10-3 Healthy implant #7 after 10 years.
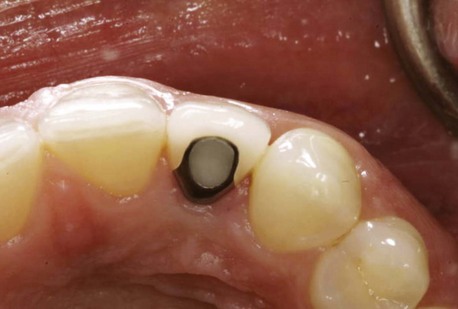
Figure 10-4 Healthy implant #7 after 10 years.
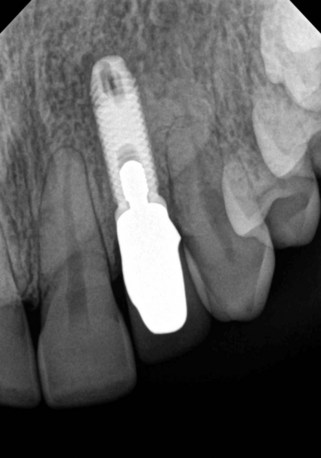
Figure 10-5 Healthy implant #7 after 10 years.
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


