Structure of metals and alloys
Microstructure of metals
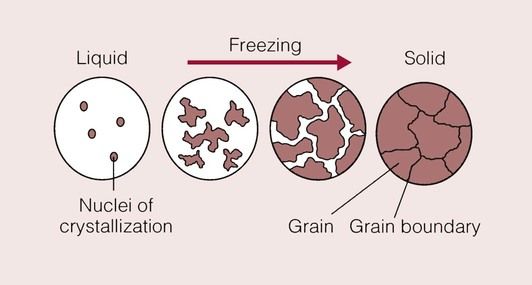
Metals consist of aggregates of atoms regularly arranged in a crystalline structure. Whereas so far we have considered the formation of single crystals, metals will not usually solidify (from what is known as the melt) as a single crystal, but instead are formed from a multitude of small crystals.
This happens because there are usually many nuclei of crystallization scattered throughout the molten metal. Such nuclei may form when four atoms lose sufficient thermal energy and become able to form a unit cell. These unit cells will grow as more metal atoms reach a low enough energy to join on, and hence crystal formation occurs. This process is known as homogeneous nucleation. It requires highly specialized equipment to grow a single crystal of metal from the entire melt.
More commonly, solidification is initiated by the presence of impurities in the melt. As the temperature drops below the melting point, metal atoms will deposit on these impurities and crystals begin to form. This process is known as heterogeneous nucleation. The crystals (or grains, as they are called) will continue to grow until all of the metal has solidified. During their growth, they will begin to impinge on one another, giving rise to boundaries between the crystals where the atoms are irregularly arranged. This boundary is called the grain boundary, and is essentially a defect in the crystal structure of the metal.
The process of solidification of a metal is shown schematically in Figure 1.4.1. A fine grain size is usually desirable in a metal because it raises the yield stress, but the reason for this will not be considered now. One way in which to promote a finer grain size is rapid solidification, as used in the casting of dental gold alloys into an investment mould that is held at a temperature well below the melting temperature of the alloy. Alternatively, the presence of many nucleating sites will give rise to a fine grain size. This method is also employed in dental gold alloys by the addition of iridium. The iridium provides many sites for nucleation and acts as a grain-refining ingredient.
It is very useful to be able to study the detailed structure of metals, in terms of the sizes of the crystals, their shape and their composition, because this information can tell us a lot about the properties of the metal and how it was made. Some idea of the structure can be obtained by examining the metal surface under a light-reflecting optical microscope.
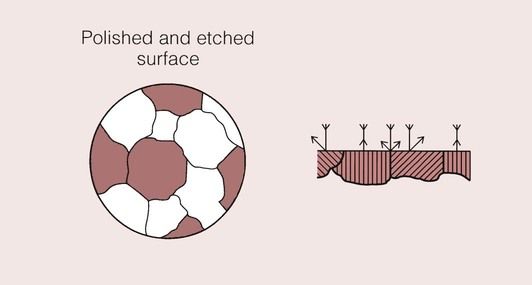
Light is reflected from a polished metal surface, but the fraction of the incident light that is reflected from any region will depend on surface irregularities, as irregularities will cause the light to be scattered.
The action of chemicals on a polished surface (known as etching) can also reduce the amount of light reflected. A suitably chosen chemical will preferentially attack certain regions of the metal surface. These areas tend to be under high local stress, such as at the grain boundaries, where there is imperfect packing of the atoms. In effect, a groove is produced that will scatter the incident light and therefore show up as a dark line.
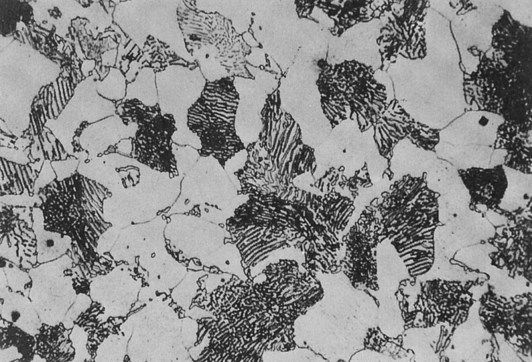
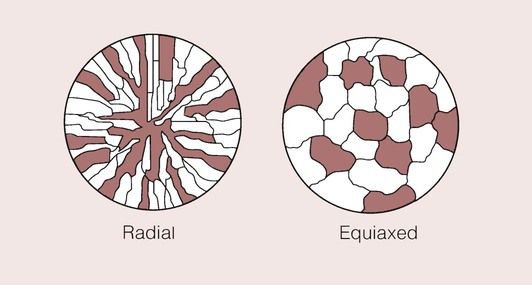
This effect is shown schematically in Figure 1.4.2 for a metal which has a very uniform grain structure. All the grains are of roughly the same size and shape; such a grain structure is described as equiaxed. An example of the grain structure for a hypo-eutectoid stainless steel, revealed by etching, is shown in Figure 1.4.3. Many other shapes and sizes of grains are possible, and these properties often depend on the methods employed during solidification. For example, if molten metal is poured into a mould with a square or circular cross-section that is held at a temperature well below the melting temperature of the metal, the grains could look something like that depicted in Figure 1.4.4. Crystal growth will have proceeded from the walls of the mould towards the centre.
Many metals are readily deformed, especially in their elemental (i.e. pure) form. This allows them to be shaped by hammering, rolling, pressing or drawing through a die. A large casting, known as an ingot, can thus be turned into any desired shape, be it a wing-panel for a car, the shell of a boat, or a wire.
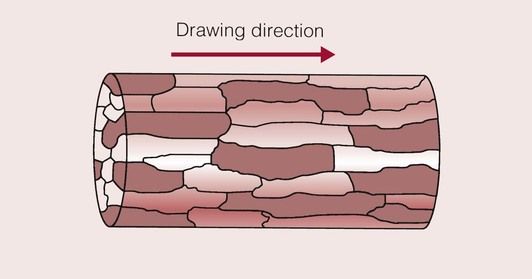
When deformed in this way, the metal is said to be wrought. If we were to examine the microstructure of a wire under the optical microscope, it would be seen to have a structure similar to that shown in Figure 1.4.5. The grains have been elongated in the direction of drawing, and have taken on a laminar structure. Thus, from looking at the microstructure of the metal we can gain a lot of information.
Alloys
Elemental metals are not generally of much use because of the severe limitations in their properties. Most metals in common use are a mixture of two or more metallic elements, sometimes with non-metallic elements included. They are usually produced by fusion of the elements above their melting temperatures. Such a mixture of two or more metals or metalloids is called an alloy. Two elements would constitute a binary alloy and a mixture of three is called a ternary alloy.
An alloy will often consist of a number of distinct solid phases, where a phase is defined as a structurally homogeneous part of the system that is separated from other parts by a definite physical boundary. Each phase will have its own distinct structure and associated properties.
The commonly cited phases are the gas, liquid and solid phases, as these are markedly different from one another. A substance can exhibit several phases.
For example, water would be considered a single-phase structure, whereas a mixture of water and oil would consist of two phases. Sand would be considered a single-phase system, even though it is made up of lots of individual particles, since each particle of sand is identical.
A phase may have more than one component – as does saline, for instance, which is an aqueous solution of sodium chloride. Similarly, phases in metals can consist of a mixture of metals. Copper can contain up to 40% zinc without destroying its FCC structure. Such a solid solution, as it is called, will satisfy some special conditions (see below).
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses