Biomaterials, safety and biocompatibility
Biomaterials
The dental restorative materials described in this textbook are a special subgroup of what are more generally known as biomaterials. When a material is placed in, or in contact with, the human body, it is generally referred to as a biomaterial. A biomaterial may be defined as a non-living material designed to interact with biological systems.
The three main areas of use of biomaterials are:
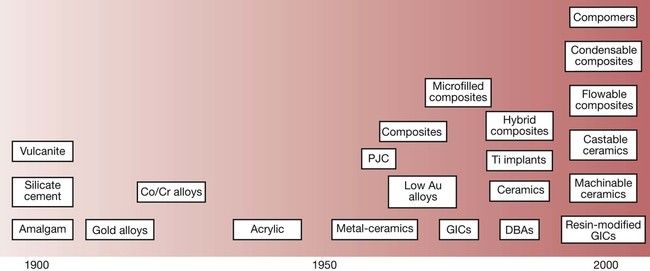
The latter part of the 20th century saw a remarkable development in new dental materials and technologies. At the beginning of the century, the choice of dental materials on offer was virtually limited to amalgam for posterior teeth, silicate cements for anterior teeth and vulcanite for dentures. At the start of the 21st century, the situation is really quite different and there is so much choice that the process of selecting the best materials for a particular clinical situation has become much more complex (Figure 1.1.1).
To make matters yet more complicated, there is now considerable pressure to make a move towards evidence-based dental practice and, by corollary, evidence-based dental material selection. However, it is not at all clear what constitutes evidence-based dental material selection, or even what constitutes evidence. If one were to start from the basis that only double-blind, randomized, controlled clinical trials constitute evidence, then with respect to dental materials we have a serious problem, as such evidence simply does not exist. So the first thing we need to do is to explore our understanding of what constitutes evidence-based dentistry more fully.
Evidence-based dentistry
There are many potential definitions of evidence-based medicine, but the one I wish to suggest as being a reasonable starting point for any discussion on this topic is that of the Centre for Evidence-Based Medicine at the University of Toronto (www.cebm.utoronto.ca), who define evidence-based medicine in the following way:
Evidence-based medicine (EBM) is the integration of best research evidence with clinical expertise and patient values.
What I like about this definition is the fact that it encompasses all aspects of the delivery of health care: namely, the evidence of research, the evidence of clinical ability, and the evidence of patient need and choice. The value of clinical ability and patient choice are reasonably easy to understand, whereas the evidence of research requires a more in-depth exploration. This is provided in the supplementary parts of the definition, which state what best research evidence is:
Clinically relevant research, often from the basic sciences of medicine, but especially from patient centered clinical research into the accuracy and precision of diagnostic tests (including the clinical examination), the power of prognostic markers, and the efficacy and safety of therapeutic, rehabilitative, and preventive regimens.
New evidence from clinical research both invalidates previously accepted diagnostic tests and treatments and replaces them with new ones that are more powerful, more accurate, more efficacious, and safer.
The important thing to point out here is the recurring theme of safety. In this book, we will concern ourselves with dental restorative materials, and a great deal of space is devoted to two important aspects of their use: their composition and their characteristic properties. However, as the evidence-based statement above clearly indicates, we must also consider the safety of patients and of dental professionals when handling dental materials.
Safety
When a biomaterial is placed in contact with the tissues and fluids of the human body, there is invariably some form of interaction between the material and the biological environment. Thus, it is quite reasonable for patients to ask their dental practitioner what evidence there is to show that the material about to be put in their mouth is safe. This does rather beg the question: ‘How do we know if a material is safe to use?’ Besides, what do we mean by ‘safe’? The most straightforward definition of safety in this context is to suggest that dental materials should not cause any local or systemic adverse reactions, either in patients or in the dental personnel handling the materials. How we might seek evidence to support the contention that the dental materials we use will not cause any adverse reactions can be gleaned from two sources, namely:
The first of these involves putting the material through a battery of laboratory experiments and testing it for cytotoxicity, mutagenicity etc., according to well-established ISO 10993 guidelines (van Loon and Mars, 1997). But that is not all, as it is important to remember that many materials have the potential to be toxic and yet can also be beneficial. For example, many chemicals used in dental materials in their raw state would be considered highly toxic (Figure 1.1.2).
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses



