Management of Soft Tissue Injuries
Initial Examination
Hemorrhage associated with most head and neck wounds may be substantial but can usually be controlled with local measures of pressure and clamping, ligation, or electrocautery of visibly bleeding vessels. Scalp wounds or disruption of major vessels may result in blood loss to the point of hypovolemic shock. According to Lynch,1 if a patient exhibits shock with facial trauma, one of three conditions is usually present: (1) the trauma is very extensive and complex, with underlying facial fractures, oropharyngeal wounds, or both and possible intracranial injury; (2) treatment has been inordinately delayed and an extended period of controlled hemorrhage or repeated episodes of bleeding have occurred; or (3) the head and neck wounds are associated with other unrecognized injuries, such as long bone fractures or chest or abdominal trauma. The face is well supplied with blood vessels, which are generally small in diameter and generously supplied with elastic fibers. When the blood vessels of the face are completely transected, they tend to contract and collapse; bleeding stops spontaneously because the vessels become occluded with thrombi and compressed by the enveloping hematoma. However, partially transected vessels have a propensity for continued hemorrhage. With an incomplete laceration of an artery, such as the facial or lingual artery, massive bleeding may occur, possibly producing compression of vital structures and potential airway compromise. Along with concomitant injury to the accompanying vein, an arteriovenous fistula may develop.
Persistent bleeding should be evaluated by direct inspection to prevent damage to other vital structures. The wound may bleed after cleansing and débridement. Copious irrigation with saline or balanced salt solutions assists with removal of blood clots and granulation tissue that may slowly ooze. Direct pressure helps control bleeding from the wound surface and limits hematoma formation. Hematoma formation is a major cause of infection and wound breakdown. If hemostasis cannot be achieved, drains should be considered.2
It is recommended before final treatment of the wounds and after cleansing of the skin that photographic records be obtained for insurance and legal purposes. Lawsuits are initiated more and more on the basis of results—not negligence—and the lay public often expects almost perfect results, whether these are realistic or not.3
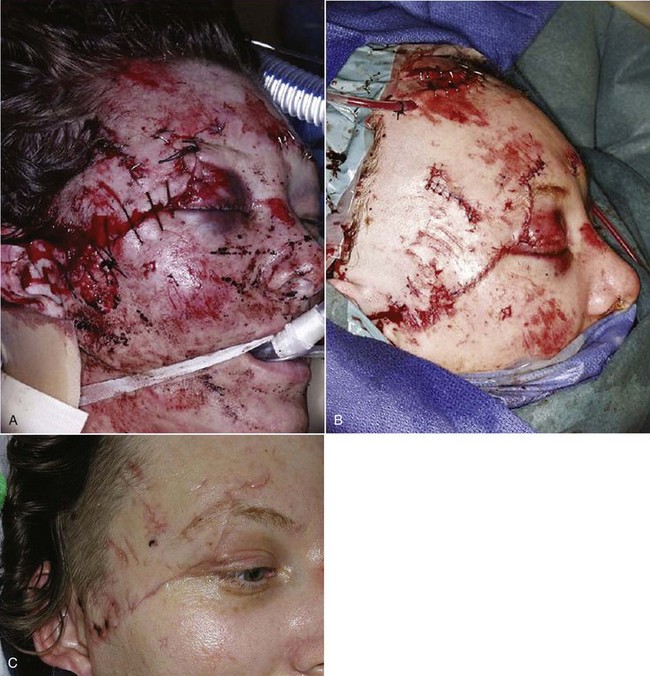
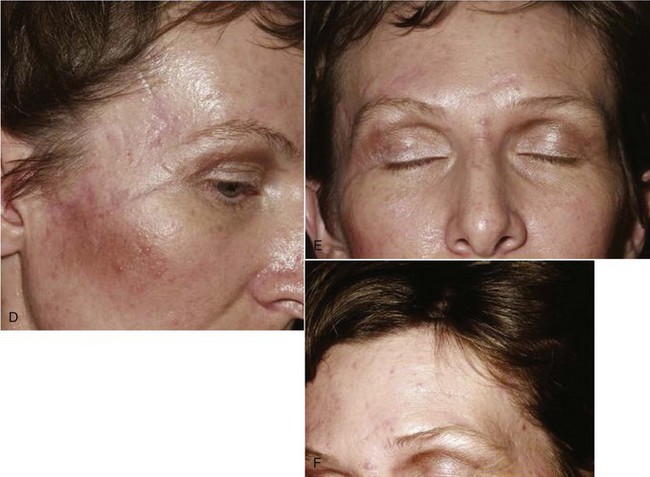
Follow-up photographs will aid in the assessment of healing and scar maturation and the necessity for future scar revision.3 After the patient’s condition has been stabilized, or if there are minimal associated injuries, definitive treatment of the soft tissue wounds should be carried out (Fig. 21-1). Clean wounds may be closed primarily up to 48 hours following injury. Healing of head wounds (nonoral) has been found to be independent of time from the injury to repair.4 Head wounds may be physiologically distinct from other types of injuries. The relatively greater vascularity of the scalp and face decreases the susceptibility of open wounds to infection. Head wounds are especially amenable to very late primary closure, days compared with the 18 to 19 hours recommended for nonhead wounds.4 This delay in the primary closure of soft tissue wounds may be indicated if the supporting facial bones have been fractured. Treatment of the fracture should be completed before final soft tissue closure because the wound may provide access to the fracture site and the closure may be damaged during fracture reduction.
Wound Contamination
Wounds can be divided into two groups, clean and contaminated. Prophylactic antibiotics are usually not indicated in clean fresh lacerations of the skin. The probability of contamination increases rapidly and is directly related to the length of time that has elapsed since the initial injury. The contamination of the clean wound is usually via Streptococcus and Staphylococcus spp. on the skin of the face and multiple types of bacteria if the mucosal layers are violated. Wounds that involve the mucosal linings of the oral cavity and pharynx, especially through and through lacerations from the skin through the mucosal layers, should be considered contaminated. Saliva may carry normal oral flora to deeper structures and wound infections may develop.3
The species of bacteria present are of less concern in the development of an infection than the total number of bacteria present within the wound. The infectious inoculum must exceed 105 organisms/g of tissue for gram-positive and gram-negative aerobic bacteria.6–8 The critical number for anaerobes has not yet been determined. Wounds such as simple lacerations and abrasions have low bacterial content. Crushing of tissue, the embedding of foreign bodies or soil, and perforation into the oral cavity with contamination of saliva markedly increase the bacterial count and set the stage for infection. Wounds caused by impact injuries are 100 times more susceptible to infection than wounds caused by shear forces.9,10
The location of the injury may be predictive of the number of pathogens in the wound. In general, the composition of the skin microflora allows for subdivision of the body into three major areas.9
High numbers of potentially infective organisms exist in the maxillofacial region and in extremely high numbers—almost double the reported infective dose—in the oral cavity.11,12 This source of heavy contamination accounts for the high infection rates from skin wounds exposed to saliva and human and animal bites.
Tetanus prophylaxis should be instituted with contaminated wounds (Table 21-1). Two thirds of reported tetanus cases in the United States in recent years have followed lacerations, puncture wounds, or crush type of injuries.10 With a previously immunized patient, if a course of active immunization has not been given within 10 years of the injury, a booster dose of 0.5 mL of tetanus toxoid is recommended. In nonimmunized patients, passive immunization with hyperimmune (human) tetanus globulin, followed by a course of active tetanus immunization, should be instituted.1,5,13 Any particularly contaminated wound should be considered for administration of tetanus prophylaxis, even though the patient may have had a booster shot in the past 5 years9,14 (Table 21-2). Antibiotics such as penicillin, cephalosporin, and other drugs active against gram-positive organisms are the drugs of choice for soft tissue injuries.
TABLE 21-1
Characteristics of Tetanus-Prone Wounds
| Clinical Features | Tetanus-Prone Wounds | Nontetanus-Prone Wounds |
| Age of wound | >6 hr | ≤6 hr |
| Configuration | Stellate wound | Linear wound |
| Depth | >1 cm | ≤1cm |
| Mechanism of injury | Missile, crush, burn, or frostbite | Sharp surface (e.g., knife) |
| Signs of infection | Present | Absent |
| Devitalized tissue | Present | Absent |
| Contaminants (dirt, feces, soil, saliva) | Present | Absent |
| Denervated or ischemic tissue, or both | Present | Absent |
TABLE 21-2
Recommendations for Tetanus Prophylaxis
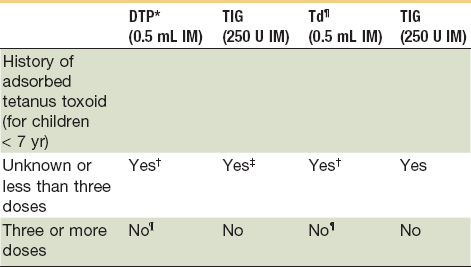
NOTE: All dog and cat wounds should be considered tetanus-prone wounds.
*Use diphtheria, tetanus (DT) if pertussis vaccine is contraindicated.
†The primary immunization series should be completed.
‡Administer in contralateral extremity.
¶Use DTP for children <7 years old (DT if pertussis vaccine is contraindicated); Td is preferred to T if patient is ≥7 years old.
¶Yes, if >5 years since last dose. (More frequent boosters are unnecessary and may accentuate side effects.)
Adapted from American College of Emergency Physicians: Tetanus immunization recommendations for persons seven years of age or older. Ann Emerg Med 15:1111, 1986; and American College of Emergency Physicians: Tetanus immunization recommendation for persons less than seven years of age. Ann Emerg Med 16:1181, 1987.
Factors that impair host resistance to infection may be classified into those that are localized to the wound and those that are systemic in the host (Box 21-1). In heavily contaminated wounds, local anesthetics with vasoconstrictor should be avoided.15
Wound Débridement
Cleansing of the clean wound involves washing the skin and removing foreign bodies from the wound. Soap does not harm the skin surface, because the thick cornified layer of epidermis protects the underlying tissue surface, but soap may enter the wound and cause cellular damage and necrosis. Toxic materials, such as alcohol, hydrogen peroxide, and benzalkonium chloride, and strong soaps, such as those containing hexachlorophene or povidone-iodine, should not have direct contact with the open wound because these materials kill cells on contact.16 If these are used around the wound, the wound should be thoroughly irrigated with a balanced salt solution (e.g., lactated Ringer’s solution, normal saline). A rule regarding the application of antiseptic is never to put anything in a wound that could not be comfortably tolerated in the conjunctival sac.17,18
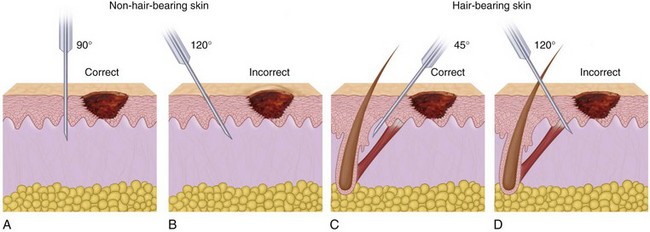
If the laceration extends into the scalp, mustache, or beard, the area should be shaved to provide good access for débridement and repair.19 Areas such as the eyebrows or the hairline should never be shaved but rather clipped closely with scissors to provide a landmark for accurate positioning of the soft tissue during the closure.20 It is important that the alignment of the eyebrow or hairline not be altered because improper orientation is aesthetically displeasing, and the hair can be used as a guide for reconstruction.
The wound must again be inspected for the presence of foreign materials. A pulsatile type of irrigating device is useful for removing debris, necrotic tissue, and loose material. An abrasive wound that contains ground dirt, glass, or other debris should be scrubbed with a scrub brush or toothbrush and detergent soap to remove the foreign material. A no. 15 blade may be used to scrape material that cannot be brushed clean or to remove deeply embedded particles, frequently seen with blast injuries. All material should be removed and time should be taken to clean the wound as completely as possible. If a contaminant is allowed to remain within the tissue, it may become a source of infection or may heal as a permanent type of tattoo that is difficult to treat with future procedures. Polymyxin B sulfate ointment may be used on the wound to remove residual grease or tar that cannot be removed with routine scrubbing techniques. The wound should then be copiously irrigated with a balanced salt solution. Time spent meticulously débriding traumatic wounds during the primary repair period will prevent unfavorable or aesthetically displeasing results from infection, hypertrophic scars, and foreign body granulomas.19,21
Wounds that have been contaminated with foreign materials, such as dirty gravel, metal, tooth fragments, grass, wood, glass, and organic materials, must be thoroughly examined during the initial treatment phase, and the material must be removed to prevent wound infections (Box 21-2). The wounds should be cleansed with detergent soaps and thoroughly irrigated with lactated Ringer’s solution or normal saline. Animal bites should be cleansed with detergents and water to remove the animal’s saliva and other contaminants from the wound before closure.
There are at least three mechanisms whereby devitalized soft tissues potentiate infection: (1) as a culture medium promoting bacterial growth; (2) by inhibition of phagocytosis and subsequent bacterial control by leukocytes; and (3) by the anaerobic environment limiting leukocyte function.22
Hydrogen peroxide should not be used routinely to cleanse a wound.23,24 Studies have shown that hydrogen peroxide actually impedes wound healing and has poor bactericidal activity. Hydrogen peroxide 3% diluted 1 : 1 does not appear to harm wounds protected by mature granulation tissue but is toxic to fibroblasts unless diluted more than 1 : 100, at which point it has minimal, if any, bactericidal activity.25 Povidone-iodine (Betadine Surgical Scrub) is also toxic to fibroblasts and bacteria. The stock solution (10% povidone-iodine, 1% available iodine) must be diluted 1 : 1000 (1 mL/liter) to prevent fibroblast toxicity. At this concentration, the bactericidal effectiveness has been compromised.
These agents should be kept out of the fresh wound and used only to scrub skin surfaces. A gauze sponge can be folded into the wound to prevent the inadvertent entry of detergents during wound healing. Mechanical scrubbing should be avoided unless there is an overwhelming amount of foreign debris in the wound. Although scrubbing can remove particulate matter from the wound, scrubbing of the wound has been shown to increase wound inflammation.24 If scrubbing of a wound is necessary, a fine pore sponge and nonionic surfactant, such as Shur-Clens, should be used to minimize the inflammatory response. When the effect of scrubbing wounds with povidone-iodine or hexachlorophene (pHisoHex) surgical scrub solutions on the infection rate was evaluated, researchers found an increased susceptibility to infection because of the greatly increased inflammatory response produced by these solutions coming into contact with the injured tissue.
In otherwise healthy patients, Dire and Welsh26 found no statistical difference in infection rates when wounds were irrigated with normal saline, 1% povidone-iodine solution, or a nonionic detergent (Shur-Clens). They noted that the mechanical action of high-pressure irrigation, not the solution used, is more important in the prevention of wound infection. Shur-Clens is a nonionic detergent that can be safely used to cleanse periorbital lacerations. Topical application of this agent to experimental animals and humans did not elicit ocular lesions. In contrast, Betadine, Hibiclens (an antimicrobial soap), and pHisoHex surgical scrub solutions caused notable irritation to the eyes and thus should not be used in the periorbital area.
Two groups of antiseptic agents, containing an iodophor or chlorhexidine, have shown promise for preparation of the intact skin around the wound. Both types exhibit activity against a broad spectrum of organisms. They also have a long shelf life, with no significant inactivation. They display a substantive effect on the skin membrane, suppressing the proliferation of bacteria. However, the superiority of one antiseptic over another is difficult to ascertain because most of the comparative studies involve hand washing rather than washing of the operative site. Although these agents can reduce the bacterial concentration of the skin, they appear to damage wound defenses and invite the development of infection. Consequently, inadvertent spillage of these agents into the wound should be avoided.9,23
Spillage of an antiseptic solution into a patient’s eye can be disastrous. It has been reported in two patients that accidental exposure to Hibiclens resulted in severe and permanent corneal opacification. In experimental studies, rabbit eyes exposed to Hibiclens developed severe irreversible and progressive corneal damage.9,27
Irrigation can remove enough wound bacteria to cross the threshold to noninfected wounds, but only if the irrigant is delivered with sufficiently high pressure to disrupt bacterial adherence to the wound surface mechanically. To be clinically effective, irrigants should be delivered with a fluid jet impacting on the wound with psi of 7 lb. This level of pressure can be generated by forcefully expressing saline from a 35-mL syringe through an 18-gauge needle, but cannot be generated by a bulb syringe or by gravity flow irrigation.28–30
Rapid and complete invasion of the wound space by fibroblasts is a critical step in normal healing. Dead tissue fragments, hematomas, and foreign bodies act as physical barriers to fibroblast penetration.17 Débridement of facial wounds should be limited to obviously devitalized and necrotic tissue. Radical excision of soft tissue in the facial region should be avoided. Because of the rich blood supply to the region, excessive débridement is unnecessary and tissue will survive with a very small pedicle. It is better to err on the side of retaining tissue that may not eventually survive than to remove tissue that is necessary for satisfactory repair of the injury. If the wound margin is extremely irregular and reapproximation is difficult, the irregular edges should be excised to produce clean wound margins and minimize scar formation. Occasionally, additional small incisions are helpful in reapproximating tissue and breaking up straight line scars.
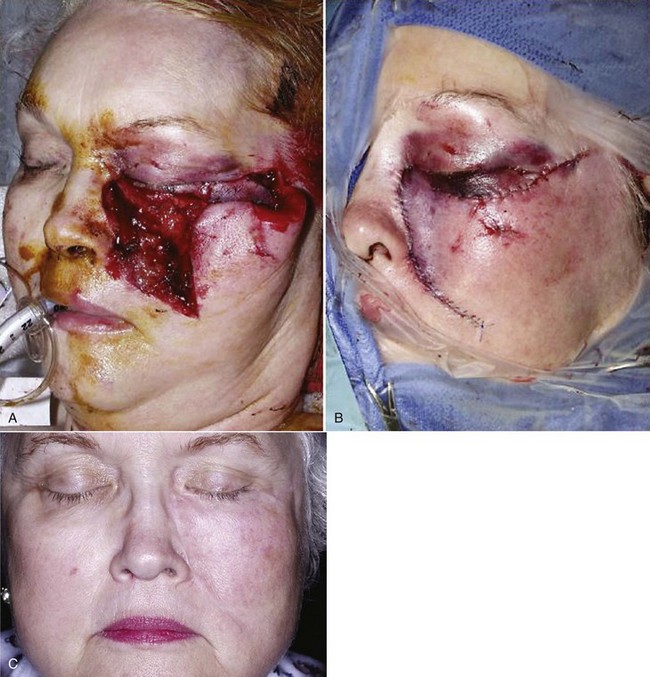
During the final examination, it is extremely important to evaluate whether vital tissue has been damaged. Deep lacerations across the course of the branches of the facial nerve and hypoglossal nerve and the sensory branch of the trigeminal nerve should be evaluated for possible transection. A nerve stimulator may be helpful in stimulating the appropriate muscle groups in the nonparalyzed patient under general anesthesia. If a nerve has been damaged, appropriate microsurgical techniques should be used to attempt to restore function of the nerve. In some avulsive injuries, secondary nerve graft procedures may be indicated. During the examination phase and initial treatment, the severed nerve trunks should be identified and marked with colored tags so that they can be easily located for future reconstruction procedures (Fig. 21-2).
Anatomy of the Skin
The skin covers the body in varying degrees of thickness, elasticity, texture, and mobility and makes transitions into mucosal membranes about the oral cavity, nostrils, and eyelids. The thickness of the skin on the facial region ranges from 0.013 inch over the upper eyelid, 0.030 to 0.040 inch over most of the face, approximately 0.065 inch over the eyebrows, and 0.080 to 0.090 inch over the neck.31
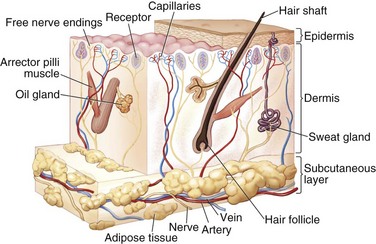
The skin is an extensive sensory organ with numerous nerve endings that provide feedback to touch, pressure, temperature, and painful stimuli (Fig. 21-3). It protects against loss of body fluids caused by dehydration, invasion of pathogenic organisms, and excessive exposure to ultraviolet radiation. The skin is also involved in temperature regulation via heat loss through evaporation.32 Subcutaneous voluntary muscles in the face and neck allow for movement of the skin and for facial expression.
The skin is composed of the surface layer epidermis and the underlying dermal layer. The epidermis is stratified squamous epithelium with five layers (in order from the surface to the dermal layer): the stratum corneum, stratum lucidum, stratum granulosum, stratum spinosum, and stratum germinativum. The epidermis sends projections into the dermis and irregularities of the dermis interlock with the epidermis; these are termed epidermal pegs and dermal papillae, respectively.32
The stratum germinativum, or basal layer, is usually one or two cells thick and has much mitotic activity.31 The basal layer is responsible for regeneration of the cells in the epidermis in the repair process and for normal turnover of cells in the epidermis. On the face, regeneration results from the germinal layer and epidermal pegs. Because of the large number of epidermal pegs on the face, a notable portion of the epidermal layer can be removed without significant scarring.33 The stratum spinosum, or prickle cell layer, consists of polyhedral cells with ovoid nuclei. The granular layer is named for its histidine-rich cytoplasmic granules of keratohyalin, thought to be important in keratin formation.34 Changes in the formation of the granular cell layer are seen in the development of the healing wound. The stratum lucidum is found only on the palms of the hands and soles of the feet. The stratum corneum, the outermost layer of the epidermis, is formed of keratinized flattened cells that are usually without nuclei. The corneum layer is responsible for the variable thicknesses of skin found on the body.
The dermis is divided into two layers, a superficial papillary layer and a deeper reticular layer. The papillary zone is a thin, finely textured zone immediately beneath the epidermal rete ridges. The papillary layer gives rise to the dermal papillae with fine fibrils of collagen and provides a blood supply to the avascular epidermal layer.32 The papillary dermis and epidermis together form a functional unit that provides an important metabolic area for retaining the normal integrity of the skin.33,34
The reticular layer of the dermis is a thick dense mass of collagenous and elastic connective tissue fibers. Reticular fibers, which give the layer its name, are young, finely formed collagen fibers with a narrower diameter than that of mature collagen.34 Elastic and other collagen fibers in the papillary dermal layer tend to be perpendicularly oriented to the overlying epidermal layer, and the fibers in the reticular layer are mainly oriented tangentially to the epidermal layer.32 Collagen fibers provide the skin with tensile strength, whereas elastic fibers give the skin its elastic properties.
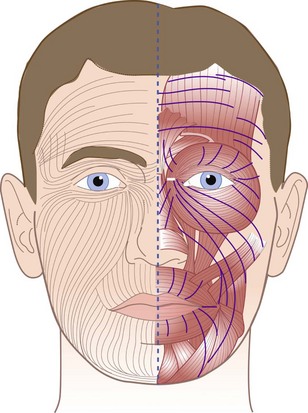
The orientation of the fibers in the reticular layer and their relationship to the epidermal layer create lines of tension in the skin that are greater in a plane perpendicular to the fibers of the reticular layer than in a plane parallel to these fibers. The predominant orientation of the fiber bundles in relation to the surface differs in different regions of the body. These patterns were described by Langer in 1861.35 He punched holes in the skin of cadavers and noted the direction of the gape of the wound, indicating the line of tension. Langer’s lines run parallel to the principal fiber bundles of the reticular layer and thus produce less tension on the wound margins (Fig. 21-4).
Langer’s lines usually indicate the most favorable direction for surgical incisions on the skin, except in some areas of the facial region because of the close relationship between the muscles of facial expression and the skin. The most inconspicuous scars are those that fall within natural creases or wrinkle lines in the skin.3 When the facial muscles contract, they produce tension on the skin in a direction perpendicular to that of the muscle group. Thus, favorable crease lines for surgical incisions on the face run parallel to the muscles of facial expression; they may not coincide with Langer’s lines and, in some areas, such as the upper lip, may run perpendicular to them.20
The dermis also contains a small amount of fat, numerous blood vessels, lymphatics, nerves and sensory nerve endings, hair follicles, sweat and sebaceous glands, and smooth muscle. The dermis is supported by subcutaneous connective tissue that is thinner in the facial region than in most of the body and is nonexistent in the eyelids. The muscles of facial expression are in the subcutaneous layer and insert into the reticular layer of the dermis.32
Local Anesthesia
• Use a needle that is small (25 gauge or smaller).
• Insert the needle into the wound margin, as opposed to piercing the intact skin.
• Pass the needle through subcutaneous tissue.
• Insert the needle no more than two thirds of its length to prevent complications associated with needle breakage.
Adverse reactions to local anesthetics do occur, but fortunately are rare. Allergic reactions can usually be prevented when a thorough patient history has been taken; these reactions are usually caused by the para-aminobenzoic acid (PABA) found in ester local anesthetic solutions or by the preservative methylparaben found in the amide local anesthetic solutions.36,37 However, the vast majority of patients who claim to be allergic to local anesthetics are not truly allergic to these agents.38,39 Many patients have been incorrectly labeled as having an allergy to local anesthetics after previously experiencing dizziness, syncope, dyspnea, localized erythema, or pruritus around the time of anesthetic administration.40,41 Nonetheless, it is difficult to determine those who may truly be sensitive to a particular agent accurately. An alternative local anesthetic for patients who report local allergies is diphenhydramine (Benadryl). At a concentration of 1%, diphenhydramine has a slower onset but a comparable duration and produces anesthesia equivalent to 1% lidocaine, although it is somewhat more painful on injection.42,43 Diphenhydramine local anesthetic solution is effective for small wounds and can be infiltrated or used for regional blocks. A quantity of 1 mL or 50-mg/mL diphenhydramine solution is diluted in 5 to 10 mL of normal saline to produce a 1.0% to 0.5% solution. When the facial area is involved, a 1% solution is recommended.44 The risk of complications with the use of diphenhydramine is low, but erythema, regional edema, vesiculation, and sloughing of tissue have been reported. Although in most cases a higher concentration was used (5%), it is recommended to avoid using this agent in areas of poor collateral perfusion such as the digits, pinnae of the ears, and nose. Common adverse reactions to diphenhydramine used as a local anesthetic also include sedation, dizziness, disturbed coordination, epigastric distress, and thickened bronchial secretions. Patients should be warned against driving or other dangerous activities because of the associated sedation.
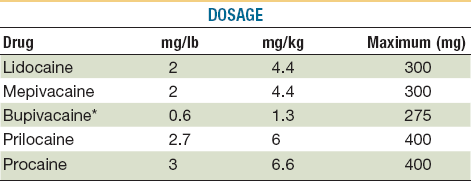
Toxic reactions to local anesthetics are more common than allergic reactions and are usually caused by accidental intravascular injection or administration of large quantities of the drug. Patients at the extremes of age are particularly at risk. Older adults can have elevated blood levels of the drug because of a decline in liver function and an increased incidence of cardiovascular disease. Overdosage is the common cause of toxicity in pediatric patients.45 Maximum dosages of all drugs administered by injection should be calculated and should not be exceeded46 (Tables 21-3 and 21-4).
TABLE 21-3
Dosages and Properties of Injectable Local Anesthetics*
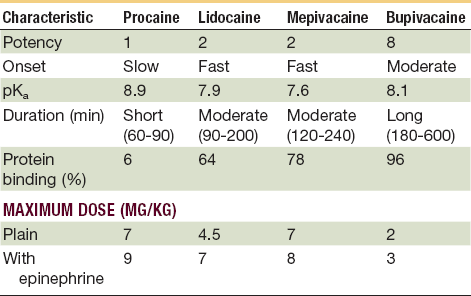
*Toxic reactions to local anesthetics are more common than allergic reactions; many are caused by accidental intravascular injection or administration of large quantities. The young and the very old are particularly at risk, so it is prudent to avoid excessive dosages and always aspirate prior to delivery.
From Webster RG, McCullough EG, Giandello PR, et al: Skin wound approximation with new absorbable suture material. Arch Otolaryngol 111:517, 1985.
The initial signs and symptoms of toxicity caused by local anesthetics are mediated primarily by the central nervous system. Initially, the patient may experience slurred speech, shivering, muscular twitching in the face and distal extremities, flushing of the skin, dizziness, tinnitus, and disorientation. Numbness of the tongue and perioral tissue is not mediated by the central nervous system. Rather, the paresthesia is caused by the direct anesthetic effect on the free nerve endings in this tissue. Further elevations of local anesthetic blood levels produce seizure activity and later cardiac and respiratory depression, which can lead to death.46 The lack of understanding about maximum dosages has led to fatalities in children.45
Vasoconstrictors can limit plasma levels of local anesthetics by decreasing the rate of absorption, which reduces the risk of toxic reactions. Additional benefits of vasoconstrictors include increased duration of action of local anesthesia and assisting with hemostasis at the surgical field. However, the use of vasoconstrictive drugs should be avoided or kept to a minimum in patients receiving certain medications such as beta blockers, monoamine oxidase (MAO) inhibitors, and tricyclic antidepressants, or in patients with conditions such as hyperthyroidism, elevated blood pressure (systolic blood pressure greater than 200 mm Hg, diastolic blood pressure greater than 115 mm Hg), and recent cerebrovascular accident or myocardial infarction.46
For the patient’s comfort, it has been found that lidocaine buffered with sodium bicarbonate can decrease pain on injection. It is recommended that 9 mL of 1% lidocaine be mixed with 1 mL of sodium bicarbonate (44 mEq/50 mL) to provide a buffered solution for injection.47
Articaine Hydrochloride (Septocaine)
One advantage of the use of articaine anesthesia in the oral cavity is that the articaine formulation may possibly spread through hard tissue more effectively than other local anesthetics and provide infiltration anesthesia as effective as nerve block techniques. Some have claimed that buccal infiltration of articaine adjacent to a maxillary or mandibular premolar provides adequate anesthesia to remove the tooth without a palatal injection. Further investigations were carried out to evaluate the use of buccal infiltration of articaine and the elimination of palatal anesthesia injections for the routine forceps removal of teeth.48
The pharmacologic and toxic effects associated with articaine are qualitatively similar to those of other amide local anesthetics. Articaine has been associated with methemoglobinemia after IV regional anesthesia, but no reports have been published about methemoglobinemia after injection for dental anesthesia.49 In a study by Malamed et al,50 4% articaine with 1 : 100,000 epinephrine was compared with 2% lidocaine with 1 : 100,000 epinephrine. Similar effectiveness and similar rates of adverse events were noted between the two local anesthetics, but a 0.9% incidence of paresthesia in a total of 882 patients was found. Long-term paresthesia, especially of the lingual nerve during inferior alveolar nerve blocks, is a growing concern with the use of this anesthetic for routine dental procedures.51,52 One may choose to use articaine primarily for infiltration techniques and with caution for nerve blocks.
Topical Agents
A combination of tetracaine (0.5%), epinephrine (1 : 2000), and cocaine (11.8%; TAC), is available for use as a topical anesthetic agent.53–55 In wounds of the scalp and face, the degree of anesthesia is comparable with that of local infiltration with lidocaine. TAC (0.09 mL/kg) is applied to gauze or cotton balls and held in contact with the wound margin for 5 to 10 minutes or until visible blanching occurs, signaling the onset of adequate anesthesia. TAC is rapidly absorbed through the mucous membranes, eyes, and burned or denuded skin and should not come into contact with these surfaces or the patient may be at risk for severe systemic toxicity or even death. It should also be avoided in areas of end-arterial flow (e.g., the digits, tip of the nose, and pinna) because of its intense vasoconstriction. TAC is used primarily for small wounds, such as simple lacerations, and is most popular in the treatment of pediatric patients.
Suture Material
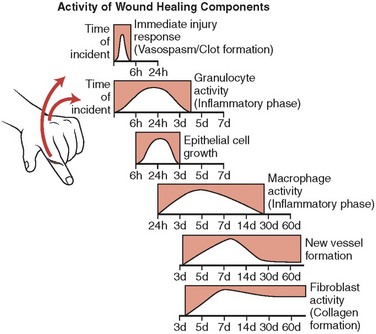
The primary purpose for using sutures is to approximate wound margins and enhance tissue healing. To understand the interaction of suture materials with biologic tissue, an appreciation of the wound-healing process is important (Fig. 21-5). Wound healing can be divided into three phases. During the initial lag phase (up to day 5), there is no gain in wound strength and the wound is dependent on sutures and epidermal cellular adhesion to maintain closure. During the fibroblastic phase (days 5 to 15), a rapid increase in wound strength occurs. The maturation phase (day 14 and beyond) is characterized by further connective tissue remodeling. By the end of the second week, when most skin sutures are removed, only 3% to 7% of the final tensile strength has been achieved. By the end of the third week, 20% of the tensile strength is attained and, at the end of the first month, 50% is present. Wounds never regain more than 80% of the strength of intact skin.56–60 Because the tissue reduces suture strength over time, the relative rates at which the suture material loses strength and the wound gains strength are important. Wounds do not gain strength until 4 to 6 days after injury, so the entire burden of approximating the tissue during this period rests on the sutures.61 Thus, tensile strength, the amount of tension or pull the suture can withstand before breaking, is an indispensable property of suture material. Tensile strength is proportional to the diameter of the suture. For example, if the diameter of the suture is doubled, the tensile strength quadruples. In addition, a knotted suture has only about one third the tensile strength of an unknotted suture, although this relationship varies with the type of knot and the suture material used.62
Suture materials should be at least as strong as the tissue in which they are used. A wide variety of materials—silk, linen, cotton, horsehair, animal tendons and intestines, and wire made of precious metals—have been used for wound closure. The evolution of suture materials has provided a number of options when selecting the correct suture for a particular procedure. The selection of suture material is based on the condition of the wound, tissue to be repaired, strength and knot-holding characteristics of the suture material, and reaction of the surrounding tissue to the suture material.3,17 Suture material can be classified as absorbable or nonabsorbable, coated or uncoated, natural or synthetic, and multifilament (braided) or monofilament. Synthetic, nonabsorbable sutures include Dacron (Mersilene, Polydek, Tevdek, Ethibond, and Tycron), nylon (Ethilon and Dermalon), and polypropylene (Prolene and Surgilene). Natural, nonabsorbable sutures include silk, cotton, and metals such as stainless steel, tantalum, and titanium. Natural absorbable sutures are made of catgut and plain and chromic collagen. Synthetic absorbable sutures include polyglycolic acid (Dexon), polyglactic acid (Vicryl), and polydioxanone (PDS).63
Monofilament sutures are made of a single strand of material. They encounter less resistance when passing through tissue than multifilament sutures and resist harboring microbial organisms. The handling properties of monofilament sutures are good, but care must be taken not to crimp or nick the material because breakage is likely. Multifilament sutures consist of several strands of material braided or wound together, which increases flexibility and tensile strength.61,64 Multifilament suture materials have been reported to cause more reactions in oral tissue compared with monofilament sutures. This increased the incidence of reactions that may be caused by permeation of the multifilament suture materials by oral bacteria. Silicone and Teflon coating of multifilament sutures does not seem to reduce bacterial invasion of tissue.65–69
Measurement of the in vivo degradation of sutures provides a general classification of surgical sutures. Sutures that undergo degradation rapidly and lose their tensile strength within 60 days are termed absorbable. Those that maintain their tensile strength for longer than 60 days are termed nonabsorbable.64,70
Absorbable Sutures
Surgical Gut Sutures
Plain gut suture is rapidly absorbed, maintaining tensile strength for only 7 to 10 days, and is completely absorbed within 70 days. Plain gut suture can also be heat treated to form fast-absorbing gut suture, which is used in epidermal suturing when support is necessary for only 5 to 7 days.70,71 Lister was the first to sterilize sutures and also introduced the treatment of catgut suture with chromic acid to slow its rate of absorption.72 To minimize tissue reaction, increase tensile strength, and slow the absorption rate of macrophage activity, catgut can be coated with a thin layer of chromium salt solution, which resists enzymatic degradation by the tissue and thus increases tensile strength and prolongs absorption time to longer than 80 days.73 In noncontaminated wounds, chromic gut sutures minimize tissue reaction, causing less irritation than plain gut in the early stages of wound healing. Tensile strength is retained for 10 to 14 days. It should also be noted that when placed into contaminated tissue, plain gut sutures elicit less infective response than chromic gut sutures.58 A mild chromic gut suture is also manufactured that is absorbed rapidly (50% in 3 to 5 days) and is used primarily in ophthalmologic surgery.62 The advantages of chromic catgut materials include absorbability, tensile strength, and knotting qualities. The disadvantages include the wide range of biologic variability in loss of tensile strength over time and a broad range of reactions to these materials in individual patients.63
A thin chromic catgut suture has been used for closure of the epidermal layer in facial wounds. The 6-0 catgut material (Davis-Geck 6-0 mild chromic, or Ethicon 6-0 rapidly absorbing gut) is absorbed within 3 to 5 days and does not have to be removed. The material may be used with sterile strips to relieve surface wound tension.3,74
Glycolic Acid Homopolymer (Dexon)
This suture, composed of a polymer of glycolic acid, is characterized by a greater knot pull and tensile strength than those of gut. It was introduced in 1970 as the first synthetic absorbable suture.75 Like Vicryl (polyglactin 910), polyglycolic acid is absorbed primarily by hydrolysis, which results in minimal tissue reactivity. However, because polyglycolic acid has been shown to persist longer in the wound, it generates more tissue reaction than Vicryl, but less than plain gut or chromic gut.76 Polyglycolic acid suture is braided and often catches on itself, making knot tying and passage through tissue difficult. The suture does not tolerate wound infections well and should not be placed at an infected site. It is also not recommended to use polyglycolic acid suture percutaneously, but this suture is effective in deeper tissue layers.
Glycolic Acid (Maxon)
Maxon is a monofilament strand composed of polyglycolic acid and trimethylene carbonate. Polyglycolic acid suture, along with PDS suture, offers the greatest tensile strength of any type of resorbable suture. The suture retains 70% of its tensile strength at 14 days and 55% at 21 days.62 This period of tensile strength is much longer than that of the chain polymer form of glycolic acid (Dexon). Complete absorption is accomplished by hydrolysis in 180 days. In vitro studies have suggested that the suspected degradation products of polyglycolic acid and nylon sutures are potent antibacterial agents. These byproducts—glycolic acid, 1,6-hexane diamine, and adipic acid—have shown a marked reduction in bacterial counts when incubated with Staphylococcus aureus.64
Polyglactin 910 (Vicryl)
This commonly used synthetic suture is composed of a mixture of lactide and glycolide acids and calcium stearate produced in a braided configuration that improves handling properties. The lactide component has hydrophobic qualities. This water-repelling property slows the penetration of water into the suture filaments, thus delaying the loss of tensile strength. Approximately 65% of its tensile strength is retained at 14 days and 40% at 21 days.77 The suture is degraded by hydrolysis and absorption is complete after 56 to 70 days, with byproducts excreted primarily in the urine. Vicryl is coated with a second type of polyglactin (polyglactin 370) and calcium stearate, which allows for easy passage through tissue and easier knot placement. This suture should be buried in the subcutaneous tissue or in deeper layers. When used in these locations, Vicryl has minimal tissue reactivity and is appropriate if the tissue is infected. However, percutaneous placement is not recommended. When used to close skin wounds, Vicryl is associated with delayed absorption and increased inflammation. Occasionally, the suture is extruded without inflammation, resulting in a small nodule in the suture line. Although this type of suture is available purple and undyed, only the colorless type should be used on the face to prevent showing through thin skin.78
Irradiated Polyglactin 910 (Vicryl Rapide)
Vicryl Rapide is irradiated polyglactin 910. It is a braided copolymer that is surface-treated with polyglactin 370 and calcium stearate and has been gamma irradiated. This radiation alters the suture material’s molecular structure and enhances its absorption rate. The suture is indicated for short-term wound support for superficial closure, providing stability of the wound for 7 to 10 days. The suture is absorbed over 12 to 14 days and does not require removal. Microscopically, the suture is absorbed primarily by phagocytosis by day 35.79,80 The degree of inflammation is less than that observed with plain or chromic catgut suture. However, Vicryl Rapide is not recommended for use on facial skin.81 The suture is slightly brittle but requires little adjustment to normal suturing techniques. From an economic standpoint, Vicryl Rapide is at most 10% more expensive than similarly packaged gut or chromic gut sutures. Polytetrafluoroethylene (PTFE) sutures are at least four times more expensive.
Vicryl Plus (Polyglactin 910 Coated with Triclosan)
In December 2002, the U.S. Food and Drug Administration (FDA) approved Vicryl Plus antibacterial suture. Designed to reduce bacterial colonization on the suture, this was the first and only suture designed with an antibacterial agent. The agent, triclosan, has been shown to be effective against S. aureus, Staphylococcus epidermidis, and methicillin-resistant strains of Staphylococcus (MRSA and MRSE), which are the leading surgical site bacteria.82 There appears to be no adverse effect on wound healing.83 The addition of triclosan also appears to have no effect on the strength, healing of wounds, handling characteristics, or performance when compared with commonly used polyglactin (Vicryl) sutures.84
Polydioxanone (PDS II)
This synthetic monofilament suture is made from the polyester derivative poly-p-dioxanone. PDS suture has excellent tensile strength qualities and retains 70% of its original tensile strength at 14 days, 50% at 28 days, and 25% at 42 days.85 The suture passes through tissue easily, but has significant memory, which compromises the ease of knot tying and knot security. Tissue reaction to the material is minimal, but there is a tendency for the PDS suture to extrude through the wound over time. Because of this tendency, it is recommended that this suture material be used only in tissue layers deeper than the subcuticular layer3 or be used, in a 6-0 size, for the closure of the epidermal layer in the face.13 PDS is commonly used in wounds under tension and is appropriate in contaminated tissue.86 Like Vicryl, PDS II is also degraded by hydrolysis. Absorption is minimum until day 90 and is complete after approximately 6 months, with minimal tissue reaction.
Nonabsorbable Sutures
Nonabsorbable sutures are categorized by the U.S. Pharmacopeia (USP)61 as follows:
• Class I: Silk or synthetic fibers of monofilaments with twisted or braided construction
• Class II: Cotton or linen fibers, coated natural or synthetic fibers in which the coating does not contribute to tensile strength
• Class III: Metal wire of monofilament or multifilament construction
Nylon
Braided (Surgilon, Nurilon).
This suture is a synthetic nonabsorbable material composed of an inert polyamide polymer. The nylon fibers are braided and then sealed with a silicone coating. Nylon has excellent knot security, tensile strength, and knot pull strength, and little tissue reactivity. The buried suture loses approximately 20% of its tensile strength yearly through hydrolysis.62 Nylon-braided sutures look, feel, and handle like silk but are stronger and do not have the increased tissue response associated with silk sutures. Multifilament nylon is weaker and less secure when knotted, offering little advantage over monofilament nylon.87
Monofilament (Dermalon, Ethilon).
The monofilament nylon suture has characteristics similar to those of the braided form of nylon suture, although it is uncoated. Monofilament nylon suture is relatively inert and nonirritating to tissue, with smooth passage through the tissue. Nylon sutures are well suited for retention and skin closure because of their elastic nature. Nylon is widely used because of its favorable qualities, such as high tensile strength and low tissue reactivity. The sutures degrade at a rate of 15% to 20% per year by hydrolysis. They have some memory and will tend to return to their original linear shape over time. Because of this tendency, more throws in the knot are indicated to securely approximate the tissue during healing, even compared with braided nylon sutures.62 Moistened nylon-monofilament sutures are more easily handled, and some types are packaged wet for use in plastic surgery procedures. A careful four-throw knot usually is sufficient.
Polyester: Braided (Tycron, Mersilene, Uncoated, Dacron, Ethibond, Coated)
Polyester sutures are constructed from multifilament fibers of polyester or polyethylene terephthalate. The polyester suture has excellent tensile strength, which is maintained indefinitely.62 Mersilene is uncoated, is somewhat rougher and stiffer than the coated form, and has a significant amount of drag when passed through the tissue. Ethibond is a braided polyester suture that is coated with polybutilate, which provides a low infection rate, secure knot tying, smooth removal, low reactivity, and easy passage through the tissue. Ethibond is an excellent suture for skin surgery; however, it is more expensive than other sutures with similar indications for use. Polyester-braided sutures are stronger than nylon or polypropylene sutures but have an increased risk of contamination and therefore are not generally used for skin closure. When used in deeper layers, the polyester suture has been shown to last indefinitely.88
Surgical Cotton
Surgical cotton is the weakest nonabsorbable suture and, as the name implies, is composed of long stable cotton fibers. Cotton suture has good knot security but is associated with high tissue drag and reactivity and has been shown to produce a marked tissue reaction.89 Surgical cotton sutures are unsuitable for use in contaminated wounds or in the presence of infection. These undesirable qualities and the fact that newer synthetic sutures provide superior performance have resulted in cotton sutures being rarely used in surgical procedures today.
Stainless Steel
Stainless steel sutures are monofilament strands of ferrous alloy that have desirable characteristics of strength and low tissue reactivity. However, this suture has the potential to corrode or break at points of twisting, bending, or knotting.90 Stainless steel suture is hard to tie and the knot ends require special handling. Both monofilament and twisted or braided multifilament stainless steel sutures are available. Stainless steel sutures offer the greatest amount of tensile strength, even in the presence of infected tissue, and are the most inert of all suture material. The difficulty in handling of stainless steel suture and its tendency to cut through tissue make it unpopular for cutaneous surgery, but it may be used successfully for closure of deeper layers that are infected.62
Silk
Silk sutures are braided, siliconized, proteinaceous thread spun of silkworm larval cocoons. Each silk filament is processed to remove the natural waxes and sericin gum. After braiding, the strands are dyed, stretched, and impregnated with a mixture of waxes and silicone. Silk sutures provide good knot security. Dry suture is stronger than wet silk suture but is not as strong as comparable sizes of synthetic materials. Silk sutures should not be used in the presence of infection.62,87 Silk sutures represent the historical and material standard of performance against which newer types of suture materials are judged, although silk sutures offer little advantage over modern synthetic materials. Silk sutures may be braided or twisted; the braided form has better handling characteristics. Silk suture is useful in the periocular area, intraorally, and on other mucosal surfaces because it remains soft and pliable and does not easily cut through tissue.3,63 Although characterized as a nonabsorbable material, studies have shown that silk sutures lose most of their tensile strength after 1 year and cannot be detected in tissue after 2 years.91
Polypropylene (Prolene)
Polypropylene suture is an isostatic crystalline stereoisomer of a linear hydrocarbon polymer permitting little or no saturation. The material is extremely inert and will retain its tensile strength for at least 2 years.92 Polypropylene suture holds knots better than most other synthetic monofilament sutures and is indicated for use when minimal suture reaction is necessary, such as infected tissue and contaminated wounds. This suture does not adhere to tissue and is flexible, and thus is useful for pull-out types of sutures. Simple knots are ineffective, but carefully tied four-throw knots will provide adequate security.87
Polybutester (Novofil)
Polybutester is a monofilament, nonabsorbable suture made of polyglycol terephthalate and polybutylene terephthalate and is considered to be a modified polyester suture.62 In contrast to polypropylene and nylon, this suture does not have significant memory, is easier to manipulate, and has greater knot security. A unique feature of polybutester sutures is their capacity to elongate or stretch with increasing wound edema. After tissue edema has subsided, the suture resumes its original shape, which theoretically makes polybutester an ideal suture material for lacerations secondary to blunt trauma.93 The tensile strength of Novofil is high and lasts for an extended period of time. Novofil has minimal tissue reactivity as well. The popularity of this suture in cutaneous surgery has been gradually increasing.
It is important to note that even the least reactive nonabsorbable suture, nylon, tends to elicit some degree of infection in tissue contaminated with Escherichia coli or S. aureus bacteria. The incidence of gross infection in contaminated tissue containing nylon sutures has been shown to be significantly greater than the infection rate in contaminated needle puncture tracks not containing suture.64 These results suggest that sutures should be avoided or the number of sutures minimized in infected tissue, whenever possible.
Surgical Tape
Microporous tape for wound closure is useful alone or in conjunction with subcutaneous or skin sutures to decrease tension at the wound margin. Skin tape comes in 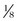 -,
-, 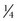 -, and
-, and 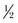 -inch wide strips that may be reinforced with rayon filaments to increase the tensile strength of the strips. The skin margin is prepared with tincture of benzoin to provide better adhesiveness for the tape. The tape should be placed perpendicular to the wound on one skin side first; the wound margins are then pulled together with the fingers or by an assistant, and the tape is secured to the skin on the other side of the wound. Thus, tension over the wound is diminished. Before placement of the tape, a thin coat of antibiotic ointment may be placed along the wound margin to protect the wound from skin oils and bacteria. To remove the adhesive tape and prevent separation of the epithelial margins, the ends should be lifted equally toward the wound margin and then lifted evenly from the wound.3
-inch wide strips that may be reinforced with rayon filaments to increase the tensile strength of the strips. The skin margin is prepared with tincture of benzoin to provide better adhesiveness for the tape. The tape should be placed perpendicular to the wound on one skin side first; the wound margins are then pulled together with the fingers or by an assistant, and the tape is secured to the skin on the other side of the wound. Thus, tension over the wound is diminished. Before placement of the tape, a thin coat of antibiotic ointment may be placed along the wound margin to protect the wound from skin oils and bacteria. To remove the adhesive tape and prevent separation of the epithelial margins, the ends should be lifted equally toward the wound margin and then lifted evenly from the wound.3
Surgical Needles
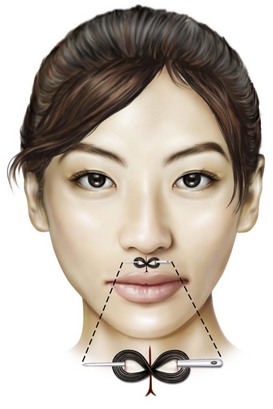
One of the earliest descriptions of needles used for surgical purposes appears in the Edwin Smith Surgical Papyrus, written approximately 3000 to 2500 BC. The twisted, or “harelip,” suture in which a needle was inserted on either side of the defect and the suture material was intertwined about the needle in a figure-eight fashion (Fig. 21-6) has been described in surgical texts published in the late 1800s, although it has commonly been assumed to be a surgical technique used in ancient times.94
Needles today are manufactured from stainless steel wire, which initially is soft and then submitted to varying heat-treating techniques to provide strength and other desirable characteristics, such as temper, hardness, malleability, and sharpness. The needles can be shaped or milled into the various types commonly used today (Fig. 21-7). Needles may be eyed or swaged. Eyed needles require threading of the suture material before use, which results in pulling a double strand of suture material through the wound and an increased risk of losing the needle in the tissue. Tying the suture to the eye is not recommended because it increases the bulk of suture material drawn through the tissue. Swaged needles do not require threading and permit a single strand of suture material to be drawn through tissue. A new and undamaged needle is provided with each strand of suture, allowing for less trauma when passed through the tissue.94,95
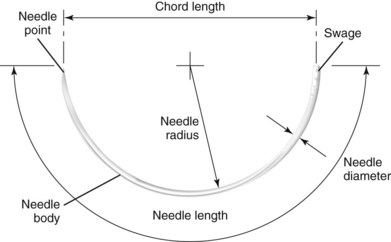
FIGURE 21-7 Anatomy of a surgical needle.
One common type of suture needle used in closing facial wounds is the reverse cutting needle (Table 21-5). The reverse curved cutting needle has a cutting edge along the convex surface, rather than along the concave surface, as in the conventional cutting needle. There is less likelihood that the needle will cut considerable tissue in its path through the tissue, and thus its use will prevent needless tissue damage and wound enlargement. It has been calculated that the strength of the needle in the reverse cutting shape is increased 32%. The increased strength of the needle makes possible the use of a smaller diameter wire, resulting in a smaller wound produced by the needle.62,94
TABLE 21-5
Characteristics of Surgical Needles Commonly Used in Skin Closure
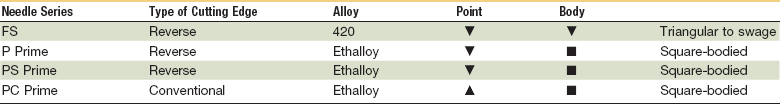
FS, For skin; P, plastic; PC, precision cosmetic; PS, plastic surgery.
Adapted from from Ethicon: Wound closure manual, Somerville, NJ, 1994, Ethicon, pp. 74-75.
The two basic varieties of needles are cuticular and plastic. Cuticular needles are sharpened 12 times, whereas plastic needles are sharpened an additional 24 times and designed to produce less trauma during penetration of tissue for cosmetic closures. Needle terminology, created by manufacturers, has developed in a haphazard manner; Ethicon currently supplies approximately 80% of the suture market. Cuticular needles may be designated as C (cuticular) or FS (for skin). Plastic-type needles may be labeled as P (for premium or plastic) or PS (plastic surgery), and PC needles are coded for precision cosmetic use. Needles in the PC series are made of a stronger stainless steel alloy and have modified, flattened, and conventional cutting profiles. A number is used to indicate the size of the needle in the various manufacturers’ series. The larger the number, the smaller the needle size in that specific series.25
Wound Closure
With repair of facial wounds, the following guidelines should be followed:
1. The injured tissue should be handled gently and minimally débrided to ensure an adequately clean bed.
2. Complete hemostasis must be obtained.
3. Incisions should be placed to follow tension lines and the natural folds of the skin.
4. The skin margins must be relaxed without tension.
5. Fine sutures should be used and removed as early as possible.
6. The wound edges should be everted.
7. Dead space must be obliterated.
8. The tissue should be closed in layers.
9. The scar tissue must be allowed to mature before revision procedures.96,97
With the closure of facial wounds, minimal tissue trauma during repair is important to prevent excessive scarring of the wound margins. Meticulous attention to detail is necessary to minimize tissue injury and prevent further scarring from poor suturing techniques. Skin hooks should be used for retraction and stabilization of tissue during débridement and repair and, when tissue forceps must be used, only those with multiple fine teeth, such as Adson-Brown forceps, should be used. Wound edges should be grasped only at the level of the subcutaneous tissue to prevent puncture marks on the skin surface.31 The wound margins should be undermined slightly to prevent undue tension on the wound margins and permit closure of the wound in layers with subcutaneous tissue and eversion of the wound margin.21 Unless necessary to elevate rotational flaps, excessive undermining of the facial tissue should be avoided to prevent unnecessary scarring and distortion of adjacent features, such as the ala of the nose, commissure of the mouth, and eyelid. Only sharp blades and scissors should be used for débridement and preparation of wound margins. Appropriate suture material on an atraumatic cutting needle is desirable for repairing facial wounds. The sutures should be placed to allow slight elevation of the wound margin, and with the tying of surgical knots it is important to remember to “approximate, not strangulate.”19
The principles of knot tying include the following:
1. Using the simplest knot that will prevent slippage
2. Tying the knot as small as possible and cutting the ends of the suture as short as reasonable to minimize foreign body reaction
3. Avoiding friction as the suture passes through the tissue (“sawing” of the tissue inevitably results in further trauma to the wound)
4. Preventing damage to the suture material that may compromise the integrity of the tied suture
5. Avoiding excessive tension that may break sutures or cut tissue
6. Approximation of the tissue-tying sutures too tightly strangulates the tissue
7. Maintaining traction at one end of the suture after the first loop is thrown to prevent loosening of the knot
8. Placing the final throw as horizontally as possible to keep the knot flat
9. Limiting extra throws to the knot, because they do not add strength to a properly tied knot
Wound closure should follow examination, débridement, and preparation of the wound margins (Fig. 21-8), if indicated, to allow meticulous alignment of the tissue. Key landmarks, such as the eyebrows, mucosal margins of the lip and nose, eyelids, and other anatomic structures, must be aligned and repaired properly. Key sutures placed to approximate these landmarks before closure of the remaining wound margins will assist in proper orientation (Fig. 21-9). Irregularities in the wound should be noted and approximated. Straight line portions of the laceration may then be closed, with the first suture placed to bisect the wound into equal sections and subsequent sutures placed in a similar fashion to provide even closure and prevent creation of so-called dog ears at the end of the wound. If dog ears develop, the sutures should be removed and closure should be attempted again; a skin hook can be inserted in the end of the wound, the tissue can be elevated, and redundant tissue can be incised around the base on one side of the wound margin.98 Every attempt should be made to accomplish closure without the necessity for removing tissue because of dog ears.
Wounds in the facial region should be repaired in layers to provide/>
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses