Dental Ceramics
Objectives
After reading this chapter, the student should be able to:
< ?mpslid E1?>< ?mpslid S2?>
< ?mpslid E2?>< ?mpslid S3?>
< ?mpslid E3?>< ?mpslid S4?>
4. Explain how the color of dental ceramics are described and assessed.
< ?mpslid E4?>< ?mpslid S5?>
5. Describe the sequence of steps in fabrication of ceramic–alloy restorations.
< ?mpslid E5?>< ?mpslid S6?>
6. Describe the process of sintering, and explain why it is important in ceramic dental restorations.
< ?mpslid E6?>< ?mpslid S7?>
< ?mpslid E7?>< ?mpslid S8?>
< ?mpslid E8?>< ?mpslid S9?>
< ?mpslid E9?>
Key Terms
Amorphous (glassy) phase
CAD-CAM
Ceramic
Ceramic inlays
Ceramic veneers
Coefficient of thermal expansion (CTE)
Condensation
Crystalline phase
Degassing
Feldspathic ceramic
Flexural strength (or transverse strength)
Fusing temperature
Hue, value, chroma
Ionic bond
Leucite
Metamerism
Opacity
Opaque
Opaquing ceramic
Porcelain
Reflectance
Sintering
Stacking
Translucent (or translucency)
Transparent (or transparency)
Veneers
Ceramic materials are fundamentally distinct from alloys or polymers; ceramics contain strong, directional, ionic bonds between metals and oxygen that impart strength but will not tolerate distortion. In dentistry, it is the interaction of light and ceramics that drive their use. Ceramics can mimic tooth esthetics better than any other material. The first use of ceramics in dentistry was for denture teeth; however, ceramic denture teeth are rarely used today because of their tendency to wear natural teeth and damage edentulous ridges (Chapter 13). But since about 1950, ceramics have been used in esthetic restorations for teeth. Because ceramics are brittle, the first ceramic-based dental restorations required that a ceramic veneer (covering) be supported by an alloy substructure. These ceramic–alloy restorations are still commonplace in today’s dental practice. Yet newer, stronger ceramics are now available to restore teeth with all-ceramic restorations, and this area of esthetic dentistry is rapidly evolving. Other roles for ceramics in dentistry include implant abutments and implants, covered in Chapter 15.
Ceramics in Dentistry
Basic Ideas about Ceramics
At the atomic level, ceramics are composed of metal-oxygen ionic bonds (see Figure 14-1). Silicon (Si), zirconium (Zr), and aluminum (Al) are common metallic elements that occur in ceramics in combination with oxygen. Ionic bonds, unlike the metallic bonds of alloys or covalent bonds of polymers, result from the complete transfer of electrons from the metal to oxygen. Ionic bonds are strong and they are directional—that is, they do not tolerate bending. This intolerance to distortion makes ceramics brittle. A second distinguishing feature of ceramics is that the metal-oxygen ionic bonds occur in vast crystalline arrays (see Figure 14-1). Metals also occur in crystalline arrays, but bonds are not ionic and are not as directional because electrons are shared (Chapter 11). Polymers also may be crystalline in nature, but the unit of the crystal is the polymer molecule, itself composed of directional covalent bonds (Chapter 13).
Ceramic crystalline arrays are not flawless, however. The continuity of the array may be interrupted by the presence of metalions of sodium (Na) or potassium (K) that cannot bond in a manner consistent with the parent metal in the array (Figure 14-2). These interrupting ions are called fluxes and have several profound effects on ceramic properties, including reduced strength, lower fusing temperature, and more transparency (see the following section on properties). When the crystalline array is disrupted sufficiently, it is described as amorphous or glassy. The latter term is used because amorphous ceramics are transparent (like window glass, which is an amorphous ceramic). The ratio between crystalline and amorphous areas in a ceramic fundamentally determines how the ceramic will behave clinically in the mouth and is the topic of the next section.
The terms ceramic and porcelain are often used interchangeably, but incorrectly. Ceramic refers to any material composed of the arrays of metallic-oxygen bonds described previously. Porcelain, on the other hand, is a type of ceramic that results when feldspar (K2O-Al2O3-SiO2), silica (SiO2), and alumina (Al2O3) are fired together with fluxes such as sodium carbonate (Na2CO3) or potassium carbonate (K2CO3). During the firing, large areas of amorphous ceramic are formed, with small islands of a crystalline phase called leucite (K[AlSi2O6]). Porcelains are often referred to as feldspathic ceramics in dentistry, and they are the most esthetic but weakest of the ceramics (see the section on properties). Feldspathic ceramics are still common in dental restorations today.
Types and Uses of Ceramics in Dentistry Today
Today’s dental ceramics may be roughly classified into four types: traditional feldspathic (or glassy), glass dominated, crystalline dominated, and crystalline (Figures 14-3 and 14-4). Each of these is discussed briefly in the text that follows, and all are a consequence of the ratio of amorphous to crystalline phases and how the phases interact with one another.
Feldspathic or glassy ceramics are porcelains that are composed primarily of an amorphous phase (often called a matrix) with embedded leucite crystals (see Figures 14-3 and 14-4). Leucite makes the porcelain more opaque, stronger, higher fusing, and creates higher expansion upon heating (see the properties section). Feldspathic ceramics also contain small amounts of metal oxides for coloring and fluorescence to optimize their esthetics. Feldspathic ceramics were used at one time for so-called porcelain jacket crowns, but because of the low strength of these ceramics, porcelain jacket crowns had high failure rates. Today, these ceramics remain among the most esthetic of the dental ceramics and are primarily used as veneers over alloys or high-strength ceramic substructures that impart strength (Table 14-1). Feldspathic ceramics also are used for veneers that are bonded directly to tooth structure, particularly in the upper anterior region.
Glass-dominated ceramics contain increased amounts of crystalline phase relative to the glassy ceramics; crystals may be leucite or fluoroapatite (see Figures 14-3 and 14-4). The increased crystalline phase gives the ceramic higher strength but sufficient translucency to serve in esthetic applications. The increased strength allows these ceramics to occasionally be used for anterior all-ceramic crowns that are not under excessive occlusal force (see Table 14-1). These materials cannot be used for posterior crowns or bridges. They are, however, excellent choices for alloy-veneering ceramics.
Crystalline-dominated ceramics are composed, as their name implies, mostly (about 70 volume %) of a crystalline phase (see Figure 14-3). The spaces between the crystals are occupied by an amorphous silica glass, and the two phases synergize to increase the strength of the ceramics substantially (see the properties section). The crystalline phase is generally spinel (MgAl2O4), zirconia (ZrO2), alumina (Al2O3), or lithium disilicate (see Figure 14-4). Crystalline-dominated ceramics are nearly opaque and therefore cannot be used by themselves as veneers or as veneers on alloys (see Table 14-1). In contrast, they often serve as cores for anterior or posterior all-ceramic crowns, onto which veneering porcelain (either glassy or glass-dominated) is added. Of the crystalline-dominated ceramics, the spinel-based ceramics have the lowest strengths, whereas the zirconia-alumina–based ceramics have the highest strengths.
The crystalline ceramics are the newest and strongest of the ceramics used in dentistry (see Figure 14-3). These ceramics are formed from either alumina or zirconia that that been seeded, or “doped,” with other ions such as magnesium or yttrium to optimize them for use in dental applications (see Figure 14-4). Crystalline ceramics have no glassy phase and are opaque. They cannot serve as esthetic veneers on alloys or teeth. However, as high-strength cores, they are plausible for use in nearly any other dental restoration, including some posterior crowns and bridges (see Table 14-1).
Fabrication Techniques
One consequence of the strong ionic bonds in ceramics is that they melt only at relatively high temperatures. For this reason, ceramic restorations are not generally cast like alloy restorations in Chapter 12 (although castable ceramics do exist). Other techniques are used to form the final dental restoration including stacking, infusing, pressing, or machining. Each of these techniques is discussed briefly in the following text.
In stacking, the parent ceramic is ground into particles that are manipulated into an approximation of the final form in a “green” state (weak state). The green state is achieved through a process called condensation (Figure 14-5). A viscous slurry of the porcelain particles in water or water-glycerol is applied to a substructure, using vibration to pack the particles and expel the liquid to the surface. The liquid is removed by a sorbent tissue. By alternatively vibrating the liquid to the surface and then removing it, the particles become packed quite tightly into the green state. In the green state, the ceramic can be carved and shaped into approximate final form. Superior condensation leads to less shrinkage during sintering (see next paragraph). Stacking is used to apply ceramics to alloys or high-strength ceramic substructures, or to fabricate laminate veneers. Stacking is used primarily for glassy and glass-dominated ceramics (see Figure 14-4).
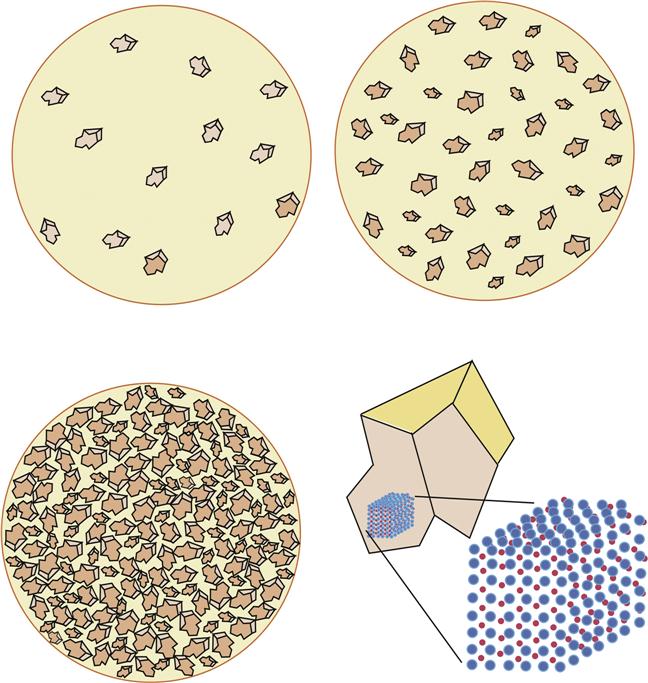
After forming the green state, the ceramic is fired in a process called sintering (Figure 14-6). During sintering, the particles only melt and fuse at their surfaces. After a short time (minutes), the particles are cooled to room temperature, and the fired ceramic is formed. To reduce the “dead” air spaces in the ceramic, a vacuum is often applied. Changes in the ratio of crystalline to amorphous phases also may occur while firing. During sintering, significant shrinkage (up to 10% linearly) occurs, and this shrinkage must be accounted for in the fabrication of a restoration. Occlusal and proximal contacts, heights of contour, and embrasures are all affected; for this reason, the green state form is often made a bit larger than final desired contours. The sintering temperature is often referred to as the fusing temperature of the ceramic.

Infusion is a fabrication method by which a porous sintered form is infiltrated with a silica glass (Figure 14-7). In the initial step, a ceramic slurry is stacked onto a refractory model of a tooth preparation, sintered to provide an intermediate strength, then trimmed to near-final form. Trimming of this intermediate form is far easier and faster than trimming the final ceramic. Next, a silica glass is added and the restoration is sintered again. During the second sintering, the silica melts and flows into all the capillary spaces of the initial structure. The infused ceramic has markedly higher strengths. Infusion is used on a subset of crystalline-dominated ceramics (see Figure 14-4).
Pressing techniques are used to force a viscous mass of molten ceramic into a mold to get the desired final form. This process is similar to casting (Chapter 12), except that the ceramic, unlike the alloy, is too viscous to accelerate into the mold and high pressure is used to move the ceramic. In addition, special investments (called refractories) are needed to survive the high temperatures of the molten ceramic; the goal is to avoid chemical reactions between the ceramic and the investment, which itself is a ceramic. Pressing has an advantage that, to some extent, the pressure can compensate for shrinkage of the ceramic and create a final form that is closer to the desired size. Pressing is often employed today for making all-ceramic cores from glass-dominated or crystalline-dominated ceramics (see Figure 14-4).
Newer ceramics are machined into final form; no condensation or sintering or melting is needed. The replica of the tooth preparation is scanned into a computer and then a highly sophisticated program drives fine machining tools to mill a ceramic block to the final form, usually in several minutes. In the most advanced techniques, the shape of the tooth preparation is captured digitally (so-called digital impression), and these data are sent to the lab for milling. Computer-aided design–computer-aided manufacturing (CAD-CAM) fabrication has several advantages over other ceramic fabrication techniques. First, because there is no heat and no condensation, shrinkage associated with stacked ceramics is not an issue (although some shrinkage is associated with ceramics milled in a green state). Second, the properties of the restoration are more predictable because manufacturing of the starting ceramic block can be tightly controlled, without the influence of a sintering or casting process to alter properties. CAD-CAM techniques are used for fabrication of high-strength ceramic cores from glass-dominated, crystalline-dominated, or crystalline ceramics (see Figure 14-4).
Properties of Ceramics
Physical Properties
As a group, ceramics exhibit extremely high compressive strengths and moduli, but relatively low tensile strengths and elongation (Table 14-2; see Chapter 2). Thus, from a clinical perspective ceramics are inherently stiff, brittle materials relative to alloys or polymers; their brittleness has limited their use in restorative dentistry over the years. The concern that a ceramic will fracture in service remains a problem for ceramic–alloy and all-ceramic restorations alike, although the newest crystalline ceramics (see Figure 14-3) are beginning to challenge this notion. Two physical properties in particular are used to assess the clinical performance of today’s dental ceramics: flexural strength and hardness, both described in the following paragraphs.
TABLE 14-2
Moduli and Strength of Ceramics versus Teeth and Common Restorative Alloys
< ?comst?>
| MATERIAL | MODULUS (GPa) | STRENGTH (MPa)∗ |
| Ceramics† | ||
| Glassy | 65–75 | 70–90‡ |
| Glass-dominated | 65–75 | 175–180 |
| Crystalline-dominated | 95 | 300–500 |
| Crystalline | 210 | 500–1200 |
| Common Restorative Alloys | ||
| TiAl6V (titanium “alloy”) | 100 | 800† |
| Stainless steel (316) | 200 | 650† |
| Cobalt-chromium | 230 | 700† |
| High-noble Au-Pd | 100 | 385† |
| Tooth | ||
| Dentin | 11 | 260† |
| Enamel | 130 | 10
Stay updated, free dental videos. Join our Telegram channel
VIDEdental - Online dental courses
 Get VIDEdental app for watching clinical videos
Get VIDEdental app for watching clinical videos

|