11
Ablative Oral/Head and Neck Surgery
INTRODUCTION
Patients undergoing benign and malignant ablative surgery of the oral cavity and head and neck region may experience a variety of medical and surgical complications perioperatively and postoperatively. These complications may be related to the surgery itself or to the patient’s preexisting physiologic compromise. Patients with a diagnosis of oral/head and neck cancer, for example, are occasionally elderly and should be considered to exhibit an immunocompromised state. Many of these patients are also malnourished. Patients with benign and malignant tumors with social habits such as smoking and medical comorbidities such as diabetes mellitus, anemia, and other diagnoses are prone to postoperative wound healing complications. As such, efforts should be made to optimize health status preoperatively and minimize perioperative and postoperative complications so as to permit patients to resume normal quality lives post ablative surgery. It is the purpose of this chapter to categorize complications associated with ablative surgery into those that occur in patients with malignant and benign tumors.
COMPLICATIONS RELATED TO ABLATIVE SURGERY FOR MALIGNANT TUMORS
Predictive Factors
Patients with malignant disease of the head and neck region are at high risk for the development of perioperative complications.1 Avoiding surgical complications in this patient cohort requires identification of those individuals with unfavorable prognostic indices such as advanced age, compromised nutritional status, and the presence of medical conditions predisposing them to higher complication rates.
Advanced Age
The effect of advanced age on surgical morbidity is a highly contested issue in the management of patients with malignant disease. One time-honored discussion showed an operative mortality of nearly 20% for patients older than 80 years of age compared to less than 5% for younger patients.2 Another study examined more than 4,300 patients undergoing orthopedic, intrathoracic, abdominal, and other surgical procedures.3 Complications were segregated according to patient decade and categorized as cardiac, such as cardiogenic pulmonary edema, myocardial infarction, unstable angina, and cardiac arrest; or as noncardiac events such as bacterial pneumonia, respiratory failure, renal failure, or pulmonary embolism. It was determined that in-hospital mortality was significantly higher in patients 80 years of age or older (2.6%) versus those patients younger than 80 years of age (0.7%). Major perioperative complications occurred in 4.3% of patients 59 years of age or younger, 5.7% of patients 60–69 years of age, 9.6% of patients 70–79 years of age, and 12.5% of patients 80 years of age or older. The authors concluded that age significantly affects the risk for perioperative cardiac and noncardiac complications after noncardiac surgery. Surgery was not prohibitive, however, in patients older than 80 years of age. In the final analysis, postoperative surgical complications such as wound infection and postoperative bleeding with hematoma formation seem to be no more common overall compared to younger patients. Medical complications, however, do seem to occur with greater frequency in older patients, including those related to a preoperative diagnosis of cardiac and pulmonary compromise.
Compromised Nutritional Status
Patients with oral cancer are often malnourished. The negative nitrogen balance noted in these patients is a reflection of poor nutrition as may exist in those with chronic alcohol abuse, inadequate oral intake due to the presence of a bulky oral tumor that may be painful, or in the case of tumor-induced weight loss.4 Surgeons have appreciated the unfavorable effects of nutritional compromise on postoperative surgical wound healing for decades, as well as the understanding that malnutrition translates to immune compromise. The observation that improved T- and B-lymphocyte mediated immune responses develop after correction of malnutrition lends credence that malnourished patients are immunocompromised.5 The prognostic nutritional index is one means to quantitatively assess the potential for cancer treatment complications. The prognostic nutritional index, as originally developed by Buzby et al.,6 uses the variables of serum albumin and transferrin values, triceps skinfold measurement, and cutaneous delayed hypersensitivity to develop a percentage value. The authors indicated that a prognostic nutritional index greater than 40% placed patients at high risk for developing treatment complications, those between 20% and 39% were at intermediate risk, and those patients with a prognostic nutritional index below 20% were at low risk of developing treatment complications. The high-risk group experienced an 89% major complication rate compared to a 12.5% major complication rate in the low-risk group. Preoperative nutritional support for longer than 7 days has been shown to decrease operative complications in patients in the high-risk group.7 While these studies were not directed at patients with oral/head and neck cancer, addressing malnutrition in this patient population preoperatively and providing chemical support of its efficacy (increased prealbumin) prior to surgery will provide a reduction in the risk of perioperative complications.
Medical Comorbidity
The traditional staging system for oral cavity and head and neck cancer is the tumor, node, metastasis (TNM) classification. This system stages cancer according to the size of the primary tumor, and the presence or absence of lymph node and distant metastatic disease. While staging systems attempt to prognosticate patients with cancers, unfortunately, their shortcoming is that they only consider the patient’s cancer and not comorbid medical conditions that may accompany the cancer and negatively affect the prognosis.8 Many patients with oral cavity and head and neck cancer have multiple medical comorbidities that can influence treatment recommendations as well as the prognosis related to ablative surgery. These comorbidities involve nearly every organ system, including the cardiac, pulmonary, endocrine, and hepatic systems. The risk of myocardial infarction or death, for example, exceeded 4% in patients with nonrevascularized coronary artery disease undergoing head and neck surgery in one study.9 Interestingly, this study identified vascular, thoracic, abdominal, and major head and neck surgery procedures as being associated with the highest risk of cardiac complications in the face of nonrevascularized coronary artery disease. The ideal method of quantifying comorbidity has not been established although several indices have been discussed in the literature including the Kaplan–Feinstein index (KFI) and the Charlson comorbidity index (CCI).10 The CCI assigns weights for diseases (Table 11.1) that are thought to alter the risk of mortality and is based on a cohort of 604 medical patients admitted to a medical service of one hospital during a 1-month period in 1984. Complete 1-year follow-up information was obtained for 559 of the 604 patients. The intention of this original report was to develop a prognostic taxonomy for comorbid conditions that singly or in combination might alter the risk of short-term mortality for patients enrolled in longitudinal studies. Results of this study indicated that the weighted index of comorbidity was a significant predictor of 1-year survival. Kim et al.1 briefly discussed unpublished data from their medical center that correlated the CCI score with survival in 117 oral cancer patients. For example, a CCI score of 2–4 showed a 5-year survival of 64% while a CCI score of 8–10 showed a 5-year survival of 14%.
Table 11.1. Weighted Index of Comorbidity
| Assigned weights for diseases | Conditions |
| 1 | Myocardial infarction |
| Congestive heart failure | |
| Peripheral vascular disease | |
| Cerebrovascular disease | |
| Dementia | |
| Chronic pulmonary disease | |
| Connective tissue disease | |
| Ulcer disease | |
| Mild liver disease | |
| Diabetes | |
| 2 | Hemiplegia |
| Moderate or severe renal disease | |
| Diabetes with end organ damage | |
| Any tumor | |
| Leukemia | |
| Lymphoma | |
| 3 | Moderate or severe liver disease |
| 6 | Metastatic solid tumor |
| AIDS |
The American Society of Anesthesiologists’ classification (ASA) of physical status has been developed for preoperative risk assessment of perioperative adverse events. This classification is not used, however, as a predictor of complications beyond the perioperative period. In addition, the main concern with the use of the ASA classification is the subjectivity involved in its assignment. Reid et al.11 assessed the ASA classification as a measure of prognosis of a cohort of head and neck surgical patients with a comparison to the Charlson index. Their study concluded that the ASA classification was slightly superior to the Charlson index for prognostic value. Clearly, the presence of comorbid medical disease impacts the prognosis of patients with oral/head and neck cancer.12
Complications
Failure to Cure
Failure to cure the disease remains the most significant and devastating negative outcome for the cancer patient and the treating team. Persistent disease, local or regional recurrence, distant metastasis, or presence of second primary cancer are among the commonest reasons for failure. The vast majority of recurrences occur within the first 2 to 3 years following completion of treatment. As it turns out, “recurrence” is a catchall term for the development of disease at the local site, the regional lymph nodes, or at distant sites. Although survival rates from treatment of squamous cell carcinoma of the oral cavity have not improved significantly over the past 30 years, the patterns of failure have changed.1 Specifically, progressive improvement in local and regional disease has been coupled with increased mortality from second primary cancers and distant metastatic disease. Local, regional, and distant failures seem to occur early following treatment for oral squamous cell carcinoma. In addition, failure is a function of stage of the cancer, particularly related to the histologic status of the cervical lymph nodes.13
Local Recurrence
Recurrence of cancer at its primary site usually represents failure to eliminate the entire cancer and achieve negative margins. In this sense, reappearance of cancer at its local site is either indicative of persistence of disease at that primary site or persistence of carcinogenic exposure to that site (Fig. 11.1). Histologically negative margins have been defined as the absence of invasive carcinoma, carcinoma in situ, and dysplasia within 5 mm of the resection margins. As such, the oncologic surgeon plans surgical ablation of oral cancers with a 1- to 1.5-cm linear margin of normal appearing tissue. The use of frozen sections of soft tissue margins in the specimen may be of assistance to this end, with a reported accuracy rate of 99%.14 Ord and Aisner14 reported a 100% recurrence rate when margins were microscopically involved by infiltrating carcinoma although Slootweg et al. reported local recurrence in 21.9% of their patients with positive margins.15 Kovacs retrospectively analyzed three subsets of patients with free or positive margins related to surgery for squamous cell carcinoma of the oral cavity and oropharynx.16 This analysis included 143 patients treated only surgically, 122 patients treated with surgery and adjuvant systemic chemotherapy, and 94 patients treated with surgery and adjuvant chemoradiation therapy. The author concluded that patients treated with adjuvant therapy following surgery with healthy margins had a survival advantage compared to those patients who underwent surgery only. Overall, disease-free survival was better in the groups with adjuvant therapy irrespective of free or positive margins. Survival rates following positive surgical margins were worse in all three groups as compared to the respective subgroups with healthy margins. A second resection in patients with positive margins who subsequently underwent chemoradiation therapy did not result in improvement in survival. The combination of healthy margins and adjuvant treatment is clearly the most favorable scenario for patient survival.
Fig. 11.1. (a) The appearance of a squamous cell carcinoma of the tongue in a 74-year-old woman who did not use alcohol or tobacco. (b) She underwent left partial glossectomy and neck dissection following diagnosis. She healed well as noted at 3 years postoperatively. (c) She displayed recurrent disease at 4 years postoperatively. A repeat partial glossectomy was performed that identified infection with human papilloma virus.
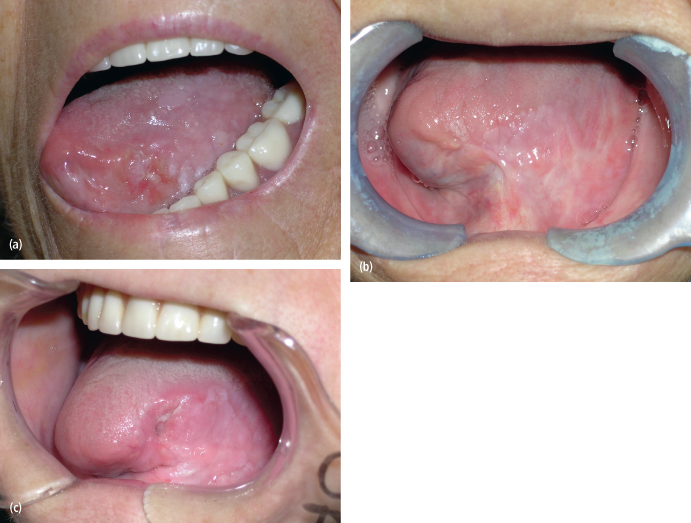
When local recurrence is noted, the time between identification of the recurrence and subsequent treatment is of prognostic significance. Schwartz et al. noted recurrent disease in 28% of their 350 patients with oral squamous cell carcinoma.17 Recurrent disease that occurred within 6 months of treatment of the primary tumor showed a mean survival time of 20 months, and no patients were cured. Recurrences occurring after 6 months resulted in a mean survival time of 58 months, and 21% of patients were salvaged with further surgery.
Regional Recurrence
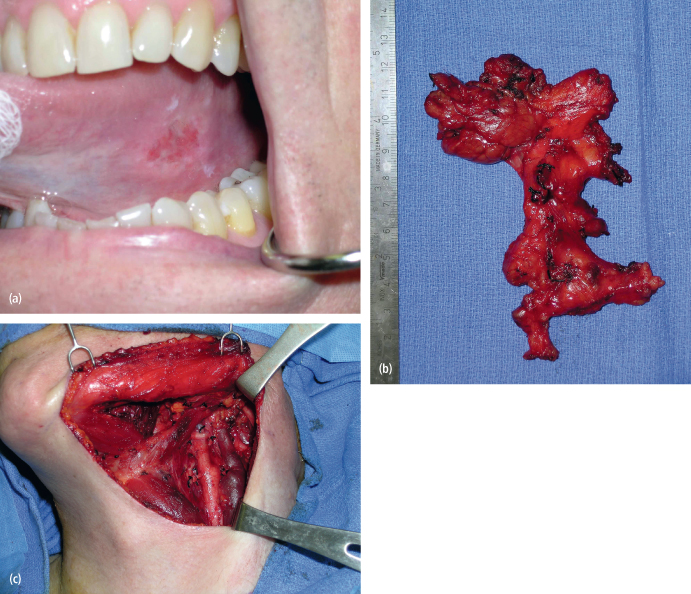
Proper execution of the prophylactic neck dissection in the absence of clinically and radiographically nodal involvement (N0 cases) is one oncologically sound method to decrease the chances of regional recurrence following surgical treatment of the primary oral cancer. This concept requires an appreciation for the presence of occult neck disease that in cancers of certain subsites (i.e., oral tongue, floor of mouth) has been reported to range between 36% and 42%.18,19 The supraomohyoid neck dissection that involves an en bloc excision of lymph nodes in levels I, II, and III represents a scientifically sound approach to the management of the N0 neck in cases of oral tongue cancers with depth of invasion greater than 3–4 mm, as well as floor of mouth lesions (Fig 11.2). In general, when the risk of occult nodal involvement is greater than 20%, the regional lymphatics require treatment. This can be in the form of surgery, selective neck dissection, or radiotherapy to the “at risk” neck. Given the low overall morbidity and complication rates of the selective neck dissection, it is the preferred approach. This is further supported by the reported poor survival (less than 50%) in cases of regional failure, especially post radiotherapy. The international literature supports the performance of this elective neck dissection for T1N0 and T2N0 squamous cell carcinomas of the oral cavity, with the identification of occult neck disease in these specimens ranging from 36% to 42%.20–22 As such, numerous authors have recommended the routine performance of the supraomohyoid neck dissection in the management of early squamous cell carcinoma of the oral cavity.23–25 Locoregional control has been reported to increase from 50% to 91% when supraomohyoid neck dissection is performed.26 A study examining 501 patients undergoing radical neck dissection showed that only 9% of patients had metastatic disease in cervical lymph nodes in level IV when the neck dissection was elective in nature and the incidence of positive lymph nodes in level V was only 2%.27 These data point to the unnecessary dissection of lymph node levels IV and V when managing the N0 neck. The only exception to this rule occurs when managing tongue cancer in which case extending the elective neck dissection into level IV is probably warranted.28
Fig. 11.2. (a) A T1N0M0 squamous cell carcinoma of the left tongue in a 60-year-old man. (b), (c) The patient underwent a left partial glossectomy and selective neck dissection (I–IV). The final histopathology of the neck specimen identified three lymph nodes with metastatic squamous cell carcinoma. The patient underwent postoperative radiation therapy and showed no evidence of disease in the neck at 3 years postoperatively.
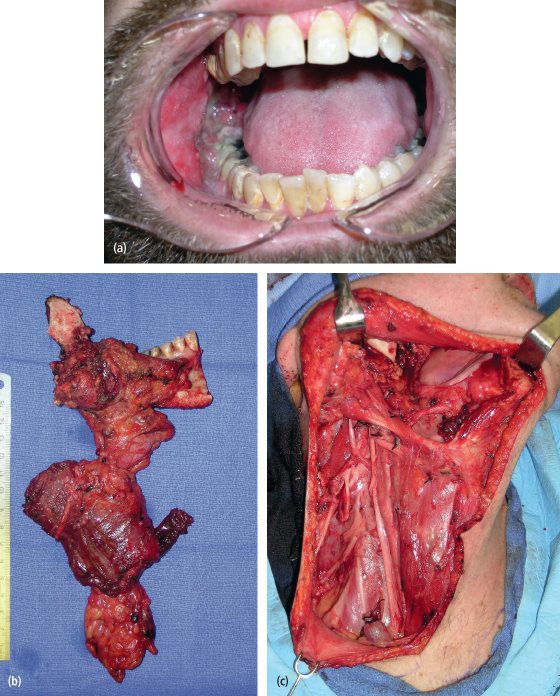
Proper execution of a neck dissection for the N+ neck also represents a method of reducing the likelihood of regional recurrences. The modified radical neck dissection and its variants are preferable in the management of the N+ neck due to the opportunity to preserve the spinal accessory nerve that otherwise would lead to a shoulder syndrome of pain and limited mobility of the upper extremity.29 The surgical principle of the modified radical neck dissection (MRND) is based on the knowledge that the aponeurotic system of the neck encases the internal structures that are routinely removed in radical neck dissection. The MRND works within these planes of dissection yet removes an en bloc specimen of metastatic and nonmetastatic lymph nodes and surrounding structures. The MRND intentionally preserves anatomic structures, most commonly the spinal accessory nerve (Fig. 11.3). For example, the type I MRND involves preservation of the spinal accessory nerve, a type II MRND involves preservation of the spinal accessory nerve and the internal jugular vein, and a type III MRND involves preservation of the spinal accessory nerve, internal jugular vein, and the sternocleidomastoid muscle. The international literature indicates that the type I MRND is the preferred neck dissection for the N+ neck in oral cavity cancers.30 This neck dissection effectively manages metastatic cervical lymph nodes, does not compromise oncologic safety, maintains function when properly performed, and should provide patients with a reduced incidence of regional recurrence depending on the final histopathology of the neck dissection specimen.13 This approach is advocated due to the observation that palpable lymph nodes even smaller than 3 cm in diameter have a substantial incidence of extracapsular spread of disease.31 The presence of extracapsular extension of cervical lymph node metastases might breech the aponeurotic planes relied upon when the sternocleidomastoid muscle and internal jugular vein are otherwise preserved. Such a surgical maneuver would likely result in recurrence in the neck postoperatively.
Fig. 11.3. (a) A 39-year-old man with a T4N1M0 squamous cell carcinoma of the right mandibular gingiva, mandible, and neck. (b), (c) He underwent a composite resection of the right mandible, gingiva, and type I modified radical neck dissection.
The functional neck dissection was originally described by Bocca and Pignataro in 1967.32 In 1984, these authors described their findings of 1,500 functional neck dissections performed in 843 patients operated on between 1961 and 1982.33 Cancer of the larynx made up 87% of the patients in this report where only lymphatic tissue of the neck was sacrificed, and the sternocleidomastoid muscle, internal jugular vein, and spinal accessory nerve were preserved. Out of these 1,500 neck dissections, 1,200 were elective in nature (N0) while 300 were therapeutic in their execution (N+). Regional recurrences were noted in 68 cases (8.1%). Sixteen cases of regional recurrence occurred in patients with N0 necks (1.33%), while 52 cases of recurrence occurred in N+ necks noted in 171 patients (30.4%). Of interest to the oral cancer surgeon is the absence of dissection of lymph nodes in level I in the functional neck dissection.34 Since level I lymph nodes are sentinel nodes related to oral cancer, failure to dissect this important oncologic level may result in cervical lymph node recurrence when managing the N0 or N+ neck. As such, it would seem that the functional neck dissection has no role in the management of cervical lymph nodes in patients with oral squamous cell carcinoma.
Distant Metastatic Disease
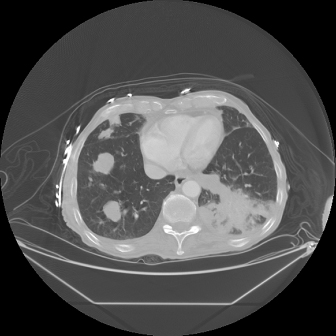
The development of distant metastatic disease related to oral cavity squamous cell carcinoma has historically been believed to represent a rare phenomenon, seen only in 2–9% of patients.35 The presence of distant metastatic disease may be disclosed by abnormal findings on a routinely ordered plain film of the chest, a PET/CT scan or a CT scan (Fig. 11.4), the presence of pain in the case of bone metastases, or the incidental discovery of metastatic foci during autopsy as may occur in a previously asymptomatic patient. Since autopsies are performed relatively infrequently following death of a patient with oral cancer, it is likely that the incidence of distant metastatic disease is underestimated.36 The most common site of distant metastatic spread has been noted as the lungs, with a mean survival time of approximately 9 months.35 The second most common distant metastatic site is bone with a mean survival time of approximately 2 months.37
Fig. 11.4. A CT of the chest in a patient treated for stage IV squamous cell carcinoma of the oral cavity 2 years earlier. The CT demonstrates evidence of metastatic disease in the right lung.
Second Primary Disease
Second primary cancers are those tumors that develop either synchronously or metach/>
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


