Fig. 14.1
Schematic representation of the MFS and ECM administration therapy as a novel therapeutic strategy for the treatment of MFS. Left panel: Fibrillin-1 comprises insoluble extracellular matrix components in connective tissue microfibrils and provides limited elasticity to tissues through fibrillin-1 microfibril formation. Missense mutations of fibrillin-1 leading to progressive connective tissue destruction due to fibrillin-1 fragmentation in association with an insufficiency of fibrillin-1 microfibril formation. ADAMTSL6β is essential for fibrillin-1 microfibril formation and suggest a novel therapeutic approach to the treatment of MFS through the promotion of ADAMTSL6β-mediated fibrillin-1 microfibril assembly. Right Panel: A variety of MFS therapies have been developed, including surgical therapy for aortic root aneurysms that are life-threatening, traditional medical therapies such as β-adrenergic receptor blockade for slow aortic growth and to decrease the risk of aortic dissection, and novel approaches based on new insights such as the deregulation of TGF-β activation. ECM reinforcement therapy which induces restoration of properly formed microfibrils by ADAMTSL6β is essential not only for improvement of the predominant symptoms of MFS, but also for the suppression of excessive TGF-β signaling induced by microfibril disassembly. Image from published paper [39]
14.4 Novel Approaches to Periodontal Tissue Regeneration Using ECM Administration Therapy
ECM components organized in the PDL not only reflect the functional requirements of this matrix such as mechanical stress and storage of signaling molecules, but also regulate the tissue framework during development and regeneration [30]. In addition, a new therapeutic concept has proposed that a fibrillin-1 microfibril insufficiency can be corrected by the administration of ECM components.
14.4.1 ADAMTSL6β Serves as a Novel Molecules that Regulate Microfibril Assembly
A disintegrin-like metalloprotease domain with thrombospondin type I motifs (ADAMTS)-like, ADAMTSL, is a subgroup of the ADAMTS superfamily that shares particular protein domains with the ADAMTS protease, including thrombospondin type I repeats, a cysteine-rich domain, and an ADAMTS spacer, but lacks the catalytic and disintegrin-like domains [40]. A recent study has demonstrated that ADAMTSL2 mutations cause geleophysic dysplasia, an autosomal recessive disorder similar to MFS, through the dysregulation of TGF-β signaling [41]. A homozygous mutation in ADAMTSL4 also causes autosomal-recessive isolated ectopia lentis, another disease similar to MFS which is characterized by the subluxation of the lens as a result of disruption of the zonular fibers [42]. The novel ADAMTSL family molecules ADAMTSL6α and 6β were recently identified by in silico screening for novel ECM proteins produced from a mouse full-length cDNA database (FANTOM). These proteins are localized in connective tissues, including the skin, aorta and perichondrocytes. Among ADAMTSL6, ADAMTSL6β has shown to associated with fibrillin-1 microfibrils through its direct interaction with the N-terminal region of fibrillin-1 and promotes fibrillin-1 matrix assembly in vitro and in vivo [43]. These findings suggest a potential clinical application of ADAMTSL6β as a novel MFS therapy by promoting fibrillin-1 microfibril assembly and regulating TGF-β activation.
It is also suggested that the administration of fibrillin-1 microfibrils provides a novel therapeutic strategy for the treatment of periodontal disease.
14.4.2 ADAMTSL6β Regulates Microfibril Assembly
To investigate whether ADAMTSL6β plays a critical role in microfibril assembly in connective tissues, we generated ADAMTSL6β transgenic mice (TSL6β-TG mice) in which the transgene is expressed in the whole body. Since ADAMTSL6β has shown to be expressed in the aorta and skin, we investigated microfibril assembly of these tissues in the TSL6β-TG mice. Immunohistochemical analysis revealed that ADAMTSL6β positive microfibril assembly was barely detectable in WT mice but strongly induced in the aorta of TSL6β-TG mice (Fig. 14.2). Histological analysis revealed that microfibrils are clearly increased in the aorta and that microfibril assembly is also induced in the skin and PDL of TSL6β − TG mice. This confirmed that ADAMTSL6β induces fibrillin-1 microfibril assembly in connective tissue such as the aorta, skin and PDL.
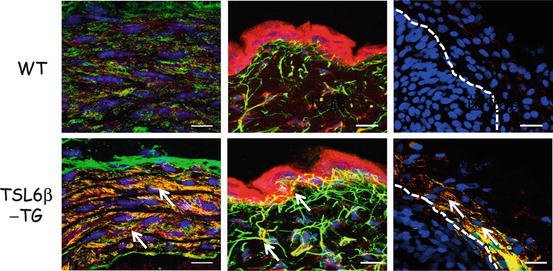

Fig. 14.2
Immunohistochemical analysis of TSL6β-TG mice. Cryosections were prepared from the aortas (left), skin (middle) or PDL (right) of wild type (upper panel) or TSL6β TG (lower panel) littermates and subjected to double immunostaining with antibodies against ADAMTSL6β (red) and fibrillin-1 (green). ADAMTSL6β and fibrillin-1-positive microfibrils (green yellow) was markedly increased in the aorta and skin of TSL6β TG mice compared with WT mice. Bar = 50 μm Image from published paper [39]
14.4.3 ADAMTSL6β Involved in PDL Formation and Repair
To investigate whether ADAMSL6β contributes to PDL formation, we first examined its expression patterns during PDL forming stage of DF in the developing tooth germ. In situ hybridization analysis revealed that ADAMSL6β was strongly expressed in the PDL forming stage of the DF however ADAMSL6β expression was significantly downregulated in the adult PDL. Immunohistochemical analysis further revealed that ADAMSL6β is detectable in assembled microfibril-like structures during the PDL forming stage of the DF, and in organized microfibrils in the adult PDL. Because developmental processes involve similar mechanisms to wound healing, we next determined whether ADAMSL6β is involved in PDL microfibril assembly during wound healing using a tooth replantation model. Histochemical analysis revealed that both fibrillin-1 and ADAMSL6β expressions were found to be clearly induced during wound healing of PDL, but to decrease again after healing. These findings suggested that ADAMSL6β was involved in microfibril formation during PDL formation/regeneration.
Since oxytalan fiber, a principal elastic fiber system of PDL is composed of fibrillin-1 microfibrils and does not contain significant amounts of elastin [44, 45], this composition suggests that PDL will have an increased susceptibility to breakdown in MFS compared with other elastic tissues composed of both elastin and fibrillin-1 [46]. We demonstrated that ADAMSL6β is highly expressed in DF during PDL forming stage. In addition, intense expression of ADAMTSL6β can be seen in wound healing process of PDL, indicating that this protein involved in recovery of damaged PDL. Using an animal model of MFS, we demonstrate that local administration of ADAMSL6β can rescue fibrillin-1 microfibril formation through the promotion of fibrillin-1 microfibril assembly in PDL (Fig. 14.3). These results strongly indicate that ADAMTSL6β is essential for fibrillin-1 microfibril formation and suggest a novel therapeutic approach to the treatment of periodontal disease with MFS.
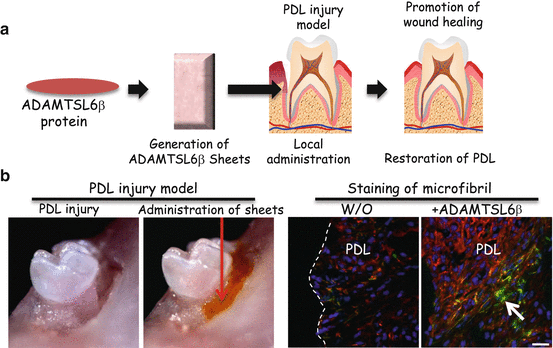

Fig. 14.3
ADAMSL6β improves microfibril disorder in PDL from an MFS model. (a) Schematic representation of the local administration of recombinant ADAMSL6β into a PDL injury model (b) After injury of PDL by dislocation, collagen gel-containing recombinant ADAMSL6β was then injected into the injured PDL (left). Immunohistochemical analysis showed an improvement in fibrillin-1 microfibril assembly (arrowheads) induced by the injection of recombinant ADAMSL6β. WO: Without treatment of ADAMSL6β. Image from published paper [17]
14.5 Conclusion
Regenerative therapy for the periodontal disease has been attempted to use of patient’s own cells to recover periodontal defect. Predictable treatment for partial regeneration of PDL damaged by local application of cytokines or stem cell trans plantation has been established, thus regenerative medicine for PDL has made the most useful study model and is feasible clinical study for the planning of stem cell- and cytokine- therapies [47]. Although partial regeneration of the periodontal tissue has been established, novel treatment must be developed corresponding to regenerate large defect destroyed by severe periodontal disease. To approach this criticism, it is essential to understand the molecular mechanisms of PDL development to identify the appropriate functional molecules of inducing differentiation of stem cells into periodontal lineage cells for successful reconstruction of periodontal tissue [17, 48, 49].
In this review, we proposed that fibrillin-1 associated protein such as ADAMTSL6β, which induces microfibril assembly, should be considered as an ECM administration agent for the treatment of periodontal disease and improvement of connective tissue disorders such as MFS. The exogenous application of recombinant ADAMTSL6β improves fibrillin-1 microfibril assembly, indicating the reinforcement of fibrillin-1 microfibrils by ADAMTSL6β may represent a new treatment for periodontal disease which is accessible from oral cavity in MFS patients. Since elastolysis occurs continuously in aortic aneurysms arising in MFS cases, the chronic administration of ADAMTSL6β may be required for the stabilization of microfibrils to prevent progressive tissue destruction. It will also be necessary to develop methodologies for the systemic administration of ADAMTSL6β to induce fibrillin-1 microfibril assembly in connective tissue for the treatment of life-threatening conditions such as an aortic aneurysm (Fig. 14.4). Hence, an ECM administration therapy involving ADAMTSL6β has the capacity to facilitate drug discovery for treating periodontal diseases, and MFS-associated disorders.

Fig. 14.4
ECM administration therapy as a novel therapeutic strategy of MFS syndrome. ECM administration therapy using ADAMTSL6β which induces microfibril assembly, should be considered in the development of future mechanism-based therapeutics for the improvement of connective tissue disorders such as MFS. Image from published paper [17]
Open Access This chapter is distributed under the terms of the Creative Commons Attribution Noncommercial License, which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


